Fda Label Requirements Medical Device . (a) except as provided by paragraphs (b) and (c) of this. Udi allows for the tracking of specific devices, and the required. Exemptions from federal preemption of state and local medical device requirements: Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. (a) the label of a device in package form shall. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Additionally, the fda requires medical device manufacturers to use unique device identification labels. Design includes labeling content that. Name and place of business of manufacturer, packer or distributor.
from mavink.com
Design includes labeling content that. Udi allows for the tracking of specific devices, and the required. Name and place of business of manufacturer, packer or distributor. (a) the label of a device in package form shall. Additionally, the fda requires medical device manufacturers to use unique device identification labels. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (a) except as provided by paragraphs (b) and (c) of this. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Exemptions from federal preemption of state and local medical device requirements:
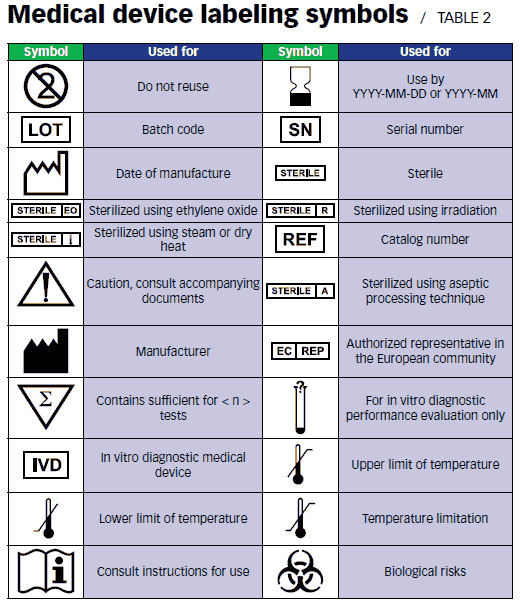
Medical Device Labeling Symbols
Fda Label Requirements Medical Device Additionally, the fda requires medical device manufacturers to use unique device identification labels. Exemptions from federal preemption of state and local medical device requirements: Udi allows for the tracking of specific devices, and the required. Design includes labeling content that. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (a) the label of a device in package form shall. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Name and place of business of manufacturer, packer or distributor. Additionally, the fda requires medical device manufacturers to use unique device identification labels. (a) except as provided by paragraphs (b) and (c) of this.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Fda Label Requirements Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Name and place of business of manufacturer, packer or distributor. Exemptions from federal preemption of state and local medical device requirements: (a) except as provided by paragraphs (b) and (c) of this. Labeling regulations pertaining to medical devices are found in the following parts. Fda Label Requirements Medical Device.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Fda Label Requirements Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Udi allows for the tracking of specific devices, and the required. (a) the label of a device in package form shall. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Additionally,. Fda Label Requirements Medical Device.
From www.vrogue.co
Fda Medical Device Labeling Regulations Archives Medi vrogue.co Fda Label Requirements Medical Device Additionally, the fda requires medical device manufacturers to use unique device identification labels. (a) except as provided by paragraphs (b) and (c) of this. Udi allows for the tracking of specific devices, and the required. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Labeling regulations pertaining to medical devices are found in. Fda Label Requirements Medical Device.
From www.schlafenderhase.com
Medical Device Labeling Requirements Schlafender Hase Fda Label Requirements Medical Device Design includes labeling content that. Udi allows for the tracking of specific devices, and the required. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Name and place of business of manufacturer, packer or distributor. (a) except as provided by paragraphs (b) and (c) of this. (a) the label of a device in. Fda Label Requirements Medical Device.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Label Requirements Medical Device Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Name and place of business of manufacturer, packer or distributor. (a) the label of a device in package form shall. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Exemptions from. Fda Label Requirements Medical Device.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Registration & Labeling Fda Label Requirements Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. (a) the label of a device in package form shall. Exemptions from federal preemption of state and local medical device requirements: Additionally, the fda requires medical device manufacturers to use unique device identification labels. Design includes labeling content that. Name and place of business. Fda Label Requirements Medical Device.
From hiveta.com
Label Compliance AB&R® (American Barcode and RFID) Fda Label Requirements Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. (a) except as provided by paragraphs (b) and (c) of this. Name and place of business of manufacturer, packer or distributor. Exemptions from federal preemption of state and local medical device requirements: (a) the label of a device in package form shall. Labeling regulations. Fda Label Requirements Medical Device.
From mavink.com
Medical Device Labeling Symbols Fda Label Requirements Medical Device Name and place of business of manufacturer, packer or distributor. Exemptions from federal preemption of state and local medical device requirements: Additionally, the fda requires medical device manufacturers to use unique device identification labels. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Design includes labeling content that. (a) the label of a. Fda Label Requirements Medical Device.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Fda Label Requirements Medical Device Udi allows for the tracking of specific devices, and the required. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (a) except as provided by paragraphs (b) and (c) of this. Design includes labeling content that. (a) the label of a device in package form shall. Additionally,. Fda Label Requirements Medical Device.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Label Requirements Medical Device Exemptions from federal preemption of state and local medical device requirements: Udi allows for the tracking of specific devices, and the required. (a) except as provided by paragraphs (b) and (c) of this. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (a) the label of a. Fda Label Requirements Medical Device.
From ar.inspiredpencil.com
Fda Labeling Regulations Fda Label Requirements Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Additionally, the fda requires medical device manufacturers to use unique device identification labels. (a) the label of a device in package form. Fda Label Requirements Medical Device.
From e-startupindia.com
US FDA labelling requirements for medical devices EStartupIndia Fda Label Requirements Medical Device Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Udi allows for the tracking of specific devices, and the required. (a) the label of a device in package form shall. Design includes labeling content that. Exemptions from federal preemption of state and local medical device requirements: Adequate. Fda Label Requirements Medical Device.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Fda Label Requirements Medical Device Udi allows for the tracking of specific devices, and the required. (a) except as provided by paragraphs (b) and (c) of this. Name and place of business of manufacturer, packer or distributor. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Design includes labeling content that. Labeling regulations pertaining to medical devices are. Fda Label Requirements Medical Device.
From hxeskyyxe.blob.core.windows.net
Fda Medical Device Label Requirements at James Day blog Fda Label Requirements Medical Device Design includes labeling content that. (a) except as provided by paragraphs (b) and (c) of this. Name and place of business of manufacturer, packer or distributor. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Udi allows for the tracking of specific devices, and the required. Labeling regulations pertaining to medical devices are. Fda Label Requirements Medical Device.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Label Requirements Medical Device Name and place of business of manufacturer, packer or distributor. Exemptions from federal preemption of state and local medical device requirements: Udi allows for the tracking of specific devices, and the required. Additionally, the fda requires medical device manufacturers to use unique device identification labels. Design includes labeling content that. Labeling regulations pertaining to medical devices are found in the. Fda Label Requirements Medical Device.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Fda Label Requirements Medical Device Udi allows for the tracking of specific devices, and the required. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (a) the label of a device in package form shall. Exemptions from federal preemption of state and local medical device requirements: Adequate labeling for a medical device. Fda Label Requirements Medical Device.
From www.tailoredlabel.com
UDI Label Requirements For FDA Medical Device Labels TLP Fda Label Requirements Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Name and place of business of manufacturer, packer or distributor. Exemptions from federal preemption of state and local medical device requirements: (a) the label of a device in package form shall. Additionally, the fda requires medical device manufacturers to use unique device identification labels.. Fda Label Requirements Medical Device.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Fda Label Requirements Medical Device Additionally, the fda requires medical device manufacturers to use unique device identification labels. Udi allows for the tracking of specific devices, and the required. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (a) the label of a device in package form shall. Design includes labeling content. Fda Label Requirements Medical Device.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Symbol Use Fda Label Requirements Medical Device (a) except as provided by paragraphs (b) and (c) of this. Design includes labeling content that. (a) the label of a device in package form shall. Additionally, the fda requires medical device manufacturers to use unique device identification labels. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Exemptions from federal preemption of. Fda Label Requirements Medical Device.
From www.greenlight.guru
Medical Device Labeling Definition & Requirements Fda Label Requirements Medical Device Design includes labeling content that. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Udi allows for the tracking of specific devices, and the required. (a) except as provided by paragraphs (b) and (c) of this. Name and place of business of manufacturer, packer or distributor. Additionally,. Fda Label Requirements Medical Device.
From www.nicelabel.com
FDA UDI compliant labelling NiceLabel Fda Label Requirements Medical Device Name and place of business of manufacturer, packer or distributor. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Exemptions from federal preemption of state and local medical device requirements: Additionally, the fda requires medical device manufacturers to use unique device identification labels. (a) the label of. Fda Label Requirements Medical Device.
From animalia-life.club
Fda Drug Labeling Requirements Fda Label Requirements Medical Device Udi allows for the tracking of specific devices, and the required. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Exemptions from federal preemption of state and local medical device requirements: Additionally, the fda requires medical device manufacturers to use unique device identification labels. Name and place. Fda Label Requirements Medical Device.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Fda Label Requirements Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Additionally, the fda requires medical device manufacturers to use unique device identification labels. Exemptions from federal preemption of state and local medical device requirements: (a) except as provided by paragraphs (b) and (c) of this. Design includes labeling content that. Labeling regulations pertaining to. Fda Label Requirements Medical Device.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical devices YouTube Fda Label Requirements Medical Device (a) except as provided by paragraphs (b) and (c) of this. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Design includes labeling content that. Exemptions from federal preemption of state and local medical device requirements: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the. Fda Label Requirements Medical Device.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Fda Label Requirements Medical Device Exemptions from federal preemption of state and local medical device requirements: Design includes labeling content that. Name and place of business of manufacturer, packer or distributor. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (a) except as provided by paragraphs (b) and (c) of this. Adequate. Fda Label Requirements Medical Device.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Fda Label Requirements Medical Device (a) the label of a device in package form shall. (a) except as provided by paragraphs (b) and (c) of this. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Exemptions from federal preemption of state and local medical device requirements: Name and place of business of. Fda Label Requirements Medical Device.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Fda Label Requirements Medical Device Design includes labeling content that. Udi allows for the tracking of specific devices, and the required. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Exemptions from federal preemption of state and local medical device requirements: Name and place of business of manufacturer, packer or distributor. Additionally,. Fda Label Requirements Medical Device.
From www.youtube.com
FDA UDI Regulation’s Impact on Medical Device Labelers inar YouTube Fda Label Requirements Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. (a) except as provided by paragraphs (b) and (c) of this. Design includes labeling content that. Exemptions from federal preemption of state and local medical device requirements: Udi allows for the tracking of specific devices, and the required. Additionally, the fda requires medical device. Fda Label Requirements Medical Device.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Registration & Labeling Fda Label Requirements Medical Device Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (a) except as provided by paragraphs (b) and (c) of this. Design includes labeling content that. Exemptions from federal preemption of state and local medical device requirements: Adequate labeling for a medical device requires proper design and procurement. Fda Label Requirements Medical Device.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Fda Label Requirements Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. (a) the label of a device in package form shall. Exemptions from federal preemption of state and local medical device requirements: Additionally, the fda requires medical device manufacturers to use unique device identification labels. Design includes labeling content that. Name and place of business. Fda Label Requirements Medical Device.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Fda Label Requirements Medical Device Udi allows for the tracking of specific devices, and the required. (a) except as provided by paragraphs (b) and (c) of this. Additionally, the fda requires medical device manufacturers to use unique device identification labels. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Adequate labeling for. Fda Label Requirements Medical Device.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Fda Label Requirements Medical Device Design includes labeling content that. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Name and place of business of manufacturer, packer or distributor. (a) the label of a device in package form shall. (a) except as provided by paragraphs (b) and (c) of this. Udi allows. Fda Label Requirements Medical Device.
From alysidia.com
21 CFR Part 801 FDA Labeling Requirements for Medical Devices Fda Label Requirements Medical Device Design includes labeling content that. Udi allows for the tracking of specific devices, and the required. Additionally, the fda requires medical device manufacturers to use unique device identification labels. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (a) the label of a device in package form. Fda Label Requirements Medical Device.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Label Requirements Medical Device (a) the label of a device in package form shall. (a) except as provided by paragraphs (b) and (c) of this. Additionally, the fda requires medical device manufacturers to use unique device identification labels. Udi allows for the tracking of specific devices, and the required. Name and place of business of manufacturer, packer or distributor. Exemptions from federal preemption of. Fda Label Requirements Medical Device.
From www.vrogue.co
Fda Medical Device Labeling Requirements Presentation vrogue.co Fda Label Requirements Medical Device Design includes labeling content that. (a) except as provided by paragraphs (b) and (c) of this. Name and place of business of manufacturer, packer or distributor. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Additionally, the fda requires medical device manufacturers to use unique device identification labels. (a) the label of a. Fda Label Requirements Medical Device.