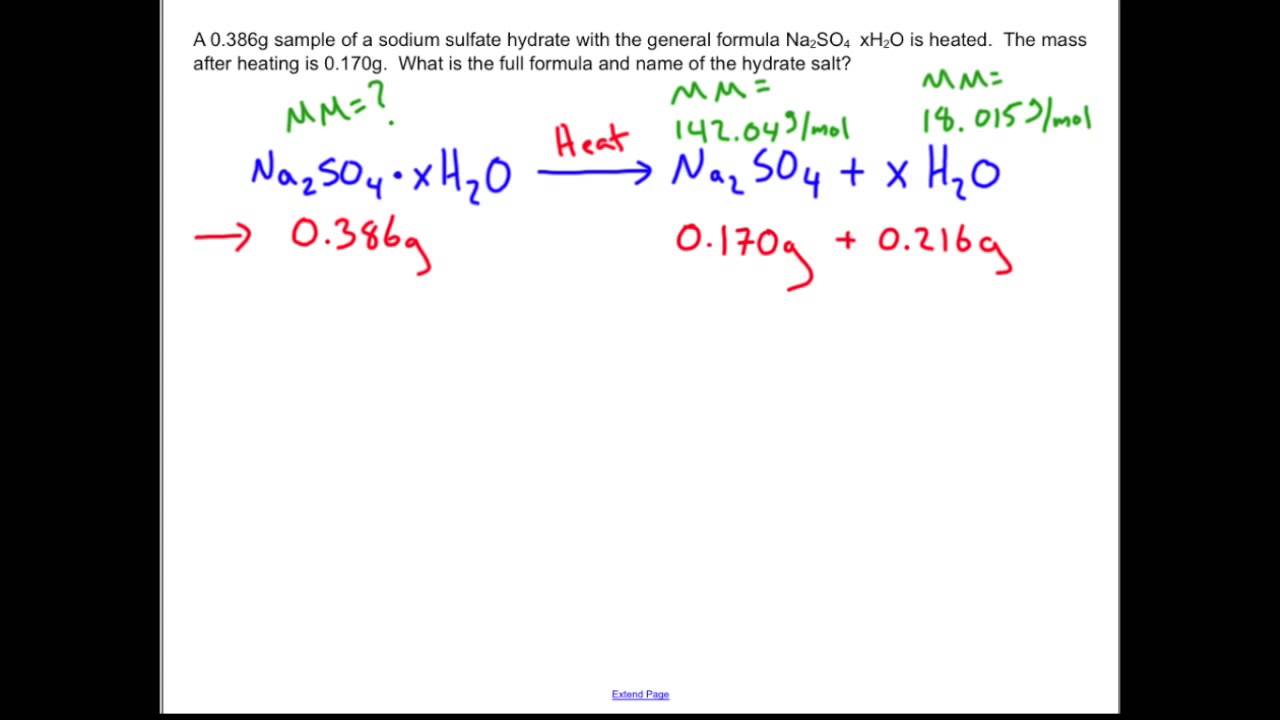
How To Use Spectrophotometer To Determine The Formula Of A Hydrate . Determine the formula of the hydrate and then write out the. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. For example, the anhydrous compound cobalt(ii) chloride is blue,. A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). After heating, the mass of the anhydrous compound is found to be 3.22 g. Use excel to generate a calibration graph. Hydrates are salts that incorporate water in their crystal struc‐ ture. In this experiment we determine the formula of a hydrate. After completing this experiment, you will be able to: In this experiment, you will determine the chemical formula for a compound that has water entrained in the crystal structure. Calculate the empirical formula of copper (ii). A hydrate can usually be converted to the anhydrous compound by heating. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a.
from materialdbbilly.z13.web.core.windows.net
Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). Calculate the empirical formula of copper (ii). Determine the formula of the hydrate and then write out the. A hydrate can usually be converted to the anhydrous compound by heating. In this experiment, you will determine the chemical formula for a compound that has water entrained in the crystal structure. In this experiment we determine the formula of a hydrate. For example, the anhydrous compound cobalt(ii) chloride is blue,. After completing this experiment, you will be able to: Use excel to generate a calibration graph.
How To Write Hydrate Formula
How To Use Spectrophotometer To Determine The Formula Of A Hydrate In this experiment we determine the formula of a hydrate. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. After heating, the mass of the anhydrous compound is found to be 3.22 g. Hydrates are salts that incorporate water in their crystal struc‐ ture. Calculate the empirical formula of copper (ii). After completing this experiment, you will be able to: A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. Determine the formula of the hydrate and then write out the. In this experiment, you will determine the chemical formula for a compound that has water entrained in the crystal structure. For example, the anhydrous compound cobalt(ii) chloride is blue,. In this experiment we determine the formula of a hydrate. Use excel to generate a calibration graph. A hydrate can usually be converted to the anhydrous compound by heating.
From www.researchgate.net
Steps involved in using the simple spectrophotometer. Download Scientific Diagram How To Use Spectrophotometer To Determine The Formula Of A Hydrate A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). In this experiment, you will determine the chemical formula for a compound that has water entrained in the crystal structure. After heating, the mass of the anhydrous compound is found to be 3.22 g. Hydrates are salts that incorporate water in. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From materialdbbilly.z13.web.core.windows.net
How To Write Hydrate Formula How To Use Spectrophotometer To Determine The Formula Of A Hydrate Use excel to generate a calibration graph. After heating, the mass of the anhydrous compound is found to be 3.22 g. Determine the formula of the hydrate and then write out the. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.youtube.com
How to Use Spectrophotometer TP 800 Color Measuring Instruments? YouTube How To Use Spectrophotometer To Determine The Formula Of A Hydrate Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. Hydrates are salts that incorporate water in their crystal struc‐ ture. After completing this experiment, you will be able to: After heating, the mass of the anhydrous compound is found to be 3.22 g. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.youtube.com
How to Use a Spectrophotometer YouTube How To Use Spectrophotometer To Determine The Formula Of A Hydrate In this experiment we determine the formula of a hydrate. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. Hydrates are salts that incorporate water in their crystal struc‐ ture. After completing this experiment, you will be able to: A hydrate contains a definite number of water molecules bound to each ionic. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.youtube.com
Spectrophotometer How To Use & Spectrophotometer Calibration YouTube How To Use Spectrophotometer To Determine The Formula Of A Hydrate Hydrates are salts that incorporate water in their crystal struc‐ ture. Determine the formula of the hydrate and then write out the. A hydrate can usually be converted to the anhydrous compound by heating. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. After heating, the mass of the anhydrous compound is. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.vrogue.co
How To Determine Formula Of Hydrate Shortcut Examples vrogue.co How To Use Spectrophotometer To Determine The Formula Of A Hydrate Use excel to generate a calibration graph. After heating, the mass of the anhydrous compound is found to be 3.22 g. Calculate the empirical formula of copper (ii). In this experiment we determine the formula of a hydrate. Hydrates are salts that incorporate water in their crystal struc‐ ture. A hydrate can usually be converted to the anhydrous compound by. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From studylib.net
Determine the formula of a hydrate How To Use Spectrophotometer To Determine The Formula Of A Hydrate After heating, the mass of the anhydrous compound is found to be 3.22 g. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). In this experiment, you will determine the chemical formula for a compound. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.pinterest.co.uk
How a Spectrophotometer works! Infographic made by Hannah Hamel Teaching chemistry, Ap How To Use Spectrophotometer To Determine The Formula Of A Hydrate In this experiment, you will determine the chemical formula for a compound that has water entrained in the crystal structure. For example, the anhydrous compound cobalt(ii) chloride is blue,. A hydrate can usually be converted to the anhydrous compound by heating. After heating, the mass of the anhydrous compound is found to be 3.22 g. Formula of a hydrate (\(\text{anhydrous. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.studocu.com
Cclab Lesson 5 HOW TO USE Spectrophotometer NCC 2023 TERM 01 MLSCC01L MIDTERM CLINICAL How To Use Spectrophotometer To Determine The Formula Of A Hydrate For example, the anhydrous compound cobalt(ii) chloride is blue,. Hydrates are salts that incorporate water in their crystal struc‐ ture. Determine the formula of the hydrate and then write out the. A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). In this experiment we determine the formula of a hydrate.. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.vrogue.co
Determine The Formula Of A Hydrate vrogue.co How To Use Spectrophotometer To Determine The Formula Of A Hydrate After completing this experiment, you will be able to: For example, the anhydrous compound cobalt(ii) chloride is blue,. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. Calculate the empirical formula of copper (ii). In this experiment, you will determine the chemical formula for a compound that has water entrained in the. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.rdworldonline.com
What are spectrophotometers? Research & Development World How To Use Spectrophotometer To Determine The Formula Of A Hydrate After completing this experiment, you will be able to: Use excel to generate a calibration graph. A hydrate can usually be converted to the anhydrous compound by heating. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a.. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.ssi.shimadzu.com
How does a spectrophotometer measure a sample? Shimadzu Scientific Instruments How To Use Spectrophotometer To Determine The Formula Of A Hydrate After completing this experiment, you will be able to: A hydrate can usually be converted to the anhydrous compound by heating. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). Determine the formula of the. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From sciencing.com
How to Use a Spectrophotometer Sciencing How To Use Spectrophotometer To Determine The Formula Of A Hydrate A hydrate can usually be converted to the anhydrous compound by heating. Determine the formula of the hydrate and then write out the. Hydrates are salts that incorporate water in their crystal struc‐ ture. For example, the anhydrous compound cobalt(ii) chloride is blue,. After heating, the mass of the anhydrous compound is found to be 3.22 g. Use excel to. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.slideserve.com
PPT Spectrophotometer PowerPoint Presentation, free download ID2341876 How To Use Spectrophotometer To Determine The Formula Of A Hydrate Calculate the empirical formula of copper (ii). In this experiment, you will determine the chemical formula for a compound that has water entrained in the crystal structure. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. A hydrate can usually be converted to the anhydrous compound by heating. Hydrates are salts that. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.vrogue.co
How To Determine Formula Of Hydrate Shortcut Examples vrogue.co How To Use Spectrophotometer To Determine The Formula Of A Hydrate In this experiment, you will determine the chemical formula for a compound that has water entrained in the crystal structure. Use excel to generate a calibration graph. After heating, the mass of the anhydrous compound is found to be 3.22 g. In this experiment we determine the formula of a hydrate. Hydrates are salts that incorporate water in their crystal. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.youtube.com
How To Use Spectrophotometer YouTube How To Use Spectrophotometer To Determine The Formula Of A Hydrate Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. Use excel to generate a calibration graph. A hydrate can usually be converted to the anhydrous compound by heating. In this experiment we determine the formula of a hydrate. In this experiment, you will determine the chemical formula for a compound that has water entrained. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.youtube.com
How to Determine Formula of Hydrate Shortcut, Examples, Practice Problem, How Many Water Formula How To Use Spectrophotometer To Determine The Formula Of A Hydrate After completing this experiment, you will be able to: Determine the formula of the hydrate and then write out the. In this experiment, you will determine the chemical formula for a compound that has water entrained in the crystal structure. A hydrate can usually be converted to the anhydrous compound by heating. For example, the anhydrous compound cobalt(ii) chloride is. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.youtube.com
Determining an Equilibrium Constant by Spectrophotometry Procedure YouTube How To Use Spectrophotometer To Determine The Formula Of A Hydrate Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. For example, the anhydrous compound cobalt(ii) chloride is blue,. Determine the formula of the hydrate and then write out the. Use excel to generate a calibration graph. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. After. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.wikihow.com
How to Do Spectrophotometric Analysis 13 Steps (with Pictures) How To Use Spectrophotometer To Determine The Formula Of A Hydrate After heating, the mass of the anhydrous compound is found to be 3.22 g. Hydrates are salts that incorporate water in their crystal struc‐ ture. Determine the formula of the hydrate and then write out the. A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). Formula of a hydrate (\(\text{anhydrous. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.chegg.com
OBJECTIVES To use a spectrophotometer to determine How To Use Spectrophotometer To Determine The Formula Of A Hydrate Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. For example, the anhydrous compound cobalt(ii) chloride is blue,. A hydrate can usually be converted to the anhydrous compound by heating. A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). In this experiment,. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.vrogue.co
How To Determine Formula Of Hydrate Shortcut Examples vrogue.co How To Use Spectrophotometer To Determine The Formula Of A Hydrate After completing this experiment, you will be able to: Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. After heating, the mass of the anhydrous compound is found to be 3.22 g. In this experiment we determine the formula of a hydrate. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.hunterlab.com
Choosing the Wavelength of Spectrophotometers How To Use Spectrophotometer To Determine The Formula Of A Hydrate After heating, the mass of the anhydrous compound is found to be 3.22 g. Calculate the empirical formula of copper (ii). Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. A hydrate can usually be converted to the anhydrous compound by heating. In this experiment, you will determine the chemical formula for. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From worksheetlistas.z19.web.core.windows.net
Determine The Formula Of A Hydrate Worksheet How To Use Spectrophotometer To Determine The Formula Of A Hydrate Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. A hydrate can usually be converted to the anhydrous compound by heating. A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). Determine the formula of the hydrate and then write out the. Hydrates are salts. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.youtube.com
Identifying Chemicals with Spectrophotometry YouTube How To Use Spectrophotometer To Determine The Formula Of A Hydrate Determine the formula of the hydrate and then write out the. Use excel to generate a calibration graph. In this experiment we determine the formula of a hydrate. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. Hydrates are salts that incorporate water in their crystal struc‐ ture. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)). How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.vrogue.co
How To Determine Formula Of Hydrate Shortcut Examples vrogue.co How To Use Spectrophotometer To Determine The Formula Of A Hydrate Calculate the empirical formula of copper (ii). After completing this experiment, you will be able to: Determine the formula of the hydrate and then write out the. In this experiment we determine the formula of a hydrate. Use excel to generate a calibration graph. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. For. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.ino.com.vn
How do you use a Spectrophotometer? A practical guide! INO Measure Co., Ltd How To Use Spectrophotometer To Determine The Formula Of A Hydrate After heating, the mass of the anhydrous compound is found to be 3.22 g. For example, the anhydrous compound cobalt(ii) chloride is blue,. Calculate the empirical formula of copper (ii). After completing this experiment, you will be able to: Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. Hydrates are salts that. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From ar.inspiredpencil.com
How A Spectrophotometer Works Diagram How To Use Spectrophotometer To Determine The Formula Of A Hydrate For example, the anhydrous compound cobalt(ii) chloride is blue,. Hydrates are salts that incorporate water in their crystal struc‐ ture. In this experiment, you will determine the chemical formula for a compound that has water entrained in the crystal structure. Use excel to generate a calibration graph. After heating, the mass of the anhydrous compound is found to be 3.22. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From meditechtips.com
How To Use a Spectrophotometer How To Use Spectrophotometer To Determine The Formula Of A Hydrate Hydrates are salts that incorporate water in their crystal struc‐ ture. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. Calculate the empirical formula of copper (ii). Use excel to generate a calibration graph. A hydrate can usually be converted to the anhydrous compound by heating. A hydrate contains a definite number of water. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.youtube.com
TRU Chemistry Labs How To Calibrate and use the Spectrophotometer YouTube How To Use Spectrophotometer To Determine The Formula Of A Hydrate Hydrates are salts that incorporate water in their crystal struc‐ ture. After completing this experiment, you will be able to: In this experiment, you will determine the chemical formula for a compound that has water entrained in the crystal structure. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. For example, the. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From schematicenginedrechsler.z19.web.core.windows.net
Circuit Diagram Of Spectrophotometer How To Use Spectrophotometer To Determine The Formula Of A Hydrate After heating, the mass of the anhydrous compound is found to be 3.22 g. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by dehydrating a. Calculate the empirical formula of copper (ii). After completing this experiment, you will be able to: In this experiment, you will determine the chemical formula for a compound that. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.biochemden.com
Spectrophotometer Instrumentation Principle and Applications How To Use Spectrophotometer To Determine The Formula Of A Hydrate Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. After completing this experiment, you will be able to: After heating, the mass of the anhydrous compound is found to be 3.22 g. Hydrates are salts that incorporate water in their crystal struc‐ ture. In this experiment, you will determine the chemical formula for a. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From clinicalsci.info
Principle of Spectrophotometer » Clinical Laboratory Science How To Use Spectrophotometer To Determine The Formula Of A Hydrate A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). A hydrate can usually be converted to the anhydrous compound by heating. Hydrates are salts that incorporate water in their crystal struc‐ ture. Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. For example, the. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From meditechtips.com
How To Use a Spectrophotometer How To Use Spectrophotometer To Determine The Formula Of A Hydrate After heating, the mass of the anhydrous compound is found to be 3.22 g. A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). After completing this experiment, you will be able to: Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. Use excel to. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.youtube.com
The Spectrophotometer Working principle, Uses, How to use guidelines) YouTube How To Use Spectrophotometer To Determine The Formula Of A Hydrate After heating, the mass of the anhydrous compound is found to be 3.22 g. A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). Formula of a hydrate (\(\text{anhydrous solid}\ce{*}x\ce{h2o}\)) the formula of a hydrate can be determined by. Hydrates are salts that incorporate water in their crystal struc‐ ture. Determine. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.
From www.vrogue.co
How Does A Spectrophotometer Work Youtube vrogue.co How To Use Spectrophotometer To Determine The Formula Of A Hydrate Calculate the empirical formula of copper (ii). Use excel to generate a calibration graph. A hydrate contains a definite number of water molecules bound to each ionic compound (also called the anhydrous salt). After completing this experiment, you will be able to: After heating, the mass of the anhydrous compound is found to be 3.22 g. Determine the formula of. How To Use Spectrophotometer To Determine The Formula Of A Hydrate.