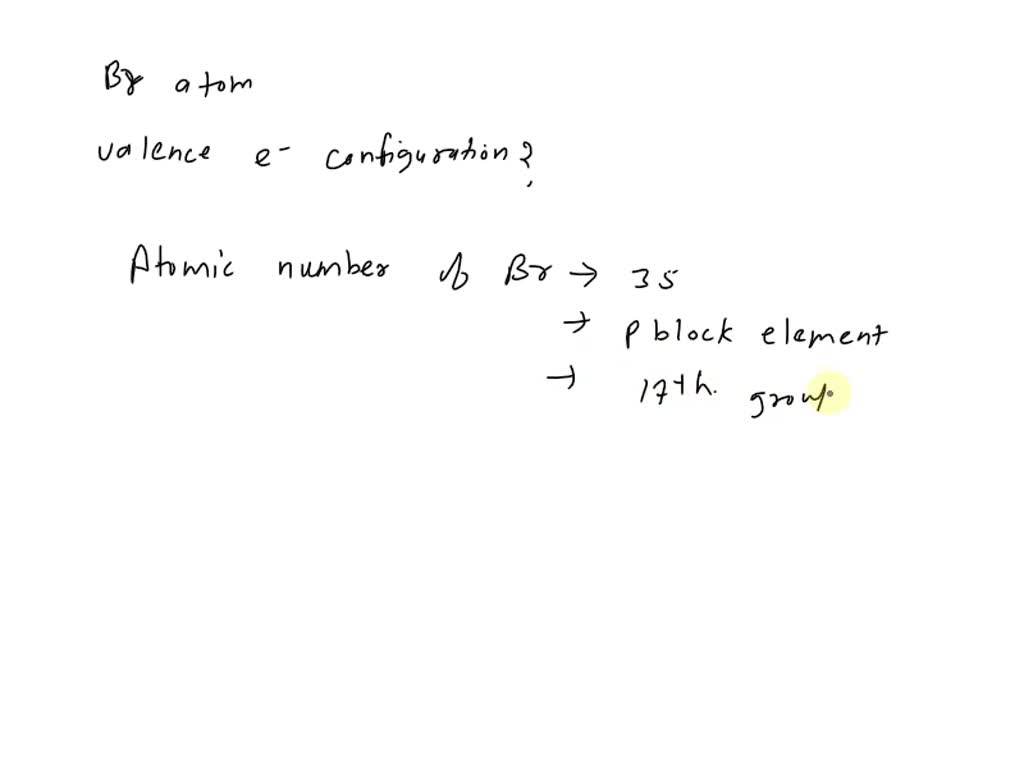
Why Doesn't Bromine Have 17 Valence Electrons . bromine (br) is a halogen and has an atomic number of 35. Bromine has seven valence electrons. Therefore, the valence electrons of. valence electrons found in the s and p orbitals of the highest energy. It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. the total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. Bromine has an electron configuration of. the valence electrons are be the 4s and 4p electrons. the electron configuration of bromine shows that the last shell of bromine has seven electrons. The electron configuration of an iron atom is 1s 2 2s 2. you may assume the valences of the chemical elements—the number of electrons with which an atom.
from www.numerade.com
Bromine has an electron configuration of. It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. you may assume the valences of the chemical elements—the number of electrons with which an atom. the total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. the electron configuration of bromine shows that the last shell of bromine has seven electrons. Bromine has seven valence electrons. bromine (br) is a halogen and has an atomic number of 35. the valence electrons are be the 4s and 4p electrons. It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. Therefore, the valence electrons of.
SOLVED What is the valence electron configuration for the bromine atom?
Why Doesn't Bromine Have 17 Valence Electrons the valence electrons are be the 4s and 4p electrons. Bromine has an electron configuration of. bromine (br) is a halogen and has an atomic number of 35. Therefore, the valence electrons of. Bromine has seven valence electrons. you may assume the valences of the chemical elements—the number of electrons with which an atom. It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. valence electrons found in the s and p orbitals of the highest energy. the total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. The electron configuration of an iron atom is 1s 2 2s 2. the electron configuration of bromine shows that the last shell of bromine has seven electrons. the valence electrons are be the 4s and 4p electrons. It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Why Doesn't Bromine Have 17 Valence Electrons the valence electrons are be the 4s and 4p electrons. you may assume the valences of the chemical elements—the number of electrons with which an atom. It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. valence electrons found in the s and p orbitals of. Why Doesn't Bromine Have 17 Valence Electrons.
From www.youtube.com
How many valence electrons does bromine have? YouTube Why Doesn't Bromine Have 17 Valence Electrons bromine (br) is a halogen and has an atomic number of 35. the valence electrons are be the 4s and 4p electrons. you may assume the valences of the chemical elements—the number of electrons with which an atom. the electron configuration of bromine shows that the last shell of bromine has seven electrons. Therefore, the valence. Why Doesn't Bromine Have 17 Valence Electrons.
From www.youtube.com
How to Find the Valence Electrons for Bromine (Br) YouTube Why Doesn't Bromine Have 17 Valence Electrons It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. the total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. Therefore, the valence electrons of. valence electrons found in the s and p orbitals of the highest energy. you. Why Doesn't Bromine Have 17 Valence Electrons.
From www.numerade.com
SOLVED What is the valence electron configuration for the bromine atom? Why Doesn't Bromine Have 17 Valence Electrons Bromine has seven valence electrons. the valence electrons are be the 4s and 4p electrons. It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. The electron configuration of an iron atom is 1s 2 2s 2. bromine (br) is a halogen and has an atomic number. Why Doesn't Bromine Have 17 Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Why Doesn't Bromine Have 17 Valence Electrons bromine (br) is a halogen and has an atomic number of 35. the electron configuration of bromine shows that the last shell of bromine has seven electrons. the valence electrons are be the 4s and 4p electrons. Therefore, the valence electrons of. Bromine has an electron configuration of. the total number of electrons present in the. Why Doesn't Bromine Have 17 Valence Electrons.
From slideplayer.com
Evaluation/ Assessment ppt download Why Doesn't Bromine Have 17 Valence Electrons It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. the electron configuration of bromine shows that the last shell of bromine has seven electrons. Bromine has an electron configuration of. The electron configuration of an iron atom is 1s 2 2s 2. valence electrons found in. Why Doesn't Bromine Have 17 Valence Electrons.
From www.slideserve.com
PPT Atoms & Chemical Bonding PowerPoint Presentation, free download Why Doesn't Bromine Have 17 Valence Electrons the total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. Bromine has seven valence electrons. the electron configuration of bromine shows that the last shell of bromine has seven electrons. It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5.. Why Doesn't Bromine Have 17 Valence Electrons.
From www.sciencecoverage.com
How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bromine] Why Doesn't Bromine Have 17 Valence Electrons Therefore, the valence electrons of. the total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. It has electronic configuration of 1s2 2s2 2p6. Why Doesn't Bromine Have 17 Valence Electrons.
From www.numerade.com
SOLVED For the Bromine atom a) Determine the total number of unpaired Why Doesn't Bromine Have 17 Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2. Therefore, the valence electrons of. Bromine has an electron configuration of. Bromine has seven valence electrons. It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. the electron configuration of bromine shows that the last shell of bromine has seven electrons. It is not. Why Doesn't Bromine Have 17 Valence Electrons.
From dxoqxvxev.blob.core.windows.net
How Many Valence Electrons Does Bromine Want at Jan Myers blog Why Doesn't Bromine Have 17 Valence Electrons the valence electrons are be the 4s and 4p electrons. valence electrons found in the s and p orbitals of the highest energy. The electron configuration of an iron atom is 1s 2 2s 2. the electron configuration of bromine shows that the last shell of bromine has seven electrons. Therefore, the valence electrons of. the. Why Doesn't Bromine Have 17 Valence Electrons.
From proper-cooking.info
Bromine Valence Electrons Why Doesn't Bromine Have 17 Valence Electrons the electron configuration of bromine shows that the last shell of bromine has seven electrons. It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. the valence electrons are be the 4s and 4p electrons. The electron configuration of an iron atom is 1s 2 2s 2.. Why Doesn't Bromine Have 17 Valence Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Why Doesn't Bromine Have 17 Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2. It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. bromine (br) is a halogen and has an atomic number of 35. Bromine. Why Doesn't Bromine Have 17 Valence Electrons.
From slideplayer.com
Chapter 8 Covalent bonding. ppt download Why Doesn't Bromine Have 17 Valence Electrons It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. bromine (br) is a halogen and has an atomic number of 35. The electron configuration of an iron atom is 1s 2 2s 2. valence electrons found in the s and p orbitals of the highest energy.. Why Doesn't Bromine Have 17 Valence Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Why Doesn't Bromine Have 17 Valence Electrons It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. the total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. bromine (br) is a halogen and has an atomic number of 35.. Why Doesn't Bromine Have 17 Valence Electrons.
From www.youtube.com
Electron Configuration of Bromine Br Lesson YouTube Why Doesn't Bromine Have 17 Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2. It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. Bromine has seven valence electrons. bromine (br) is a halogen and has an atomic number of 35. Bromine has an electron configuration of. the total number of electrons present in the valence shell. Why Doesn't Bromine Have 17 Valence Electrons.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Why Doesn't Bromine Have 17 Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2. the valence electrons are be the 4s and 4p electrons. Therefore, the valence electrons of. the electron configuration of bromine shows that the last shell of bromine has seven electrons. valence electrons found in the s and p orbitals of the highest energy. you. Why Doesn't Bromine Have 17 Valence Electrons.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Why Doesn't Bromine Have 17 Valence Electrons the total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. Bromine has seven valence electrons. valence electrons found in the s and p orbitals of the highest energy. It is not the valence electrons themselves, but the number of valence electrons that determines. Why Doesn't Bromine Have 17 Valence Electrons.
From slideplayer.com
Bell Work 9/14/17 Complete Electron Configurations worksheet 14, ppt Why Doesn't Bromine Have 17 Valence Electrons you may assume the valences of the chemical elements—the number of electrons with which an atom. bromine (br) is a halogen and has an atomic number of 35. the electron configuration of bromine shows that the last shell of bromine has seven electrons. valence electrons found in the s and p orbitals of the highest energy.. Why Doesn't Bromine Have 17 Valence Electrons.
From brainly.com
Which element has the same number of valence electrons as bromine (Br Why Doesn't Bromine Have 17 Valence Electrons Therefore, the valence electrons of. The electron configuration of an iron atom is 1s 2 2s 2. the valence electrons are be the 4s and 4p electrons. It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. valence electrons found in the s and p orbitals of. Why Doesn't Bromine Have 17 Valence Electrons.
From exobbalij.blob.core.windows.net
Bromine Atom Electrons at Paula McCullough blog Why Doesn't Bromine Have 17 Valence Electrons It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. you may assume the valences of the chemical elements—the number of electrons with which an atom. Therefore, the valence electrons of. It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. bromine (br) is. Why Doesn't Bromine Have 17 Valence Electrons.
From dxogpncsm.blob.core.windows.net
Bromine Element Protons Neutrons And Electrons at Kelly Moya blog Why Doesn't Bromine Have 17 Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2. the valence electrons are be the 4s and 4p electrons. bromine (br) is a halogen and has an atomic number of 35. It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. Bromine has seven valence electrons. valence electrons found in the. Why Doesn't Bromine Have 17 Valence Electrons.
From materialcampusmatthews.z21.web.core.windows.net
How To Identify The Valence Electron Why Doesn't Bromine Have 17 Valence Electrons Bromine has an electron configuration of. Therefore, the valence electrons of. you may assume the valences of the chemical elements—the number of electrons with which an atom. It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. the electron configuration of bromine shows that the last shell. Why Doesn't Bromine Have 17 Valence Electrons.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Why Doesn't Bromine Have 17 Valence Electrons It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. the total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. Bromine has an electron configuration of. you may assume the valences of the chemical elements—the number of electrons with which. Why Doesn't Bromine Have 17 Valence Electrons.
From www.youtube.com
How many valence electrons does bromine have? YouTube Why Doesn't Bromine Have 17 Valence Electrons Bromine has seven valence electrons. the total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. valence electrons found in the s and p orbitals of the highest energy. the electron configuration of bromine shows that the last shell of bromine has seven. Why Doesn't Bromine Have 17 Valence Electrons.
From material-properties.org
Bromine Periodic Table and Atomic Properties Why Doesn't Bromine Have 17 Valence Electrons you may assume the valences of the chemical elements—the number of electrons with which an atom. Bromine has seven valence electrons. Therefore, the valence electrons of. Bromine has an electron configuration of. valence electrons found in the s and p orbitals of the highest energy. the total number of electrons present in the valence shell of an. Why Doesn't Bromine Have 17 Valence Electrons.
From dxosghbuq.blob.core.windows.net
Electron Dot Structure For Bromine at Teresa Davis blog Why Doesn't Bromine Have 17 Valence Electrons the valence electrons are be the 4s and 4p electrons. bromine (br) is a halogen and has an atomic number of 35. It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. Bromine has. Why Doesn't Bromine Have 17 Valence Electrons.
From mavink.com
Lewis Dot Diagram For Bromine Why Doesn't Bromine Have 17 Valence Electrons It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. Therefore, the valence electrons of. Bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2. valence electrons found. Why Doesn't Bromine Have 17 Valence Electrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Why Doesn't Bromine Have 17 Valence Electrons Therefore, the valence electrons of. valence electrons found in the s and p orbitals of the highest energy. you may assume the valences of the chemical elements—the number of electrons with which an atom. the total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of. Why Doesn't Bromine Have 17 Valence Electrons.
From ar.inspiredpencil.com
Orbital Diagram Of Bromine Why Doesn't Bromine Have 17 Valence Electrons Bromine has an electron configuration of. you may assume the valences of the chemical elements—the number of electrons with which an atom. Bromine has seven valence electrons. the valence electrons are be the 4s and 4p electrons. bromine (br) is a halogen and has an atomic number of 35. the total number of electrons present in. Why Doesn't Bromine Have 17 Valence Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Why Doesn't Bromine Have 17 Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2. Therefore, the valence electrons of. you may assume the valences of the chemical elements—the number of electrons with which an atom. It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. Bromine has seven valence electrons. the total number of electrons present in. Why Doesn't Bromine Have 17 Valence Electrons.
From proper-cooking.info
Bromine Valence Electrons Why Doesn't Bromine Have 17 Valence Electrons It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. The electron configuration of an iron atom is 1s 2 2s 2. Bromine has seven valence electrons. the electron configuration of bromine shows that the last shell of bromine has seven electrons. you may assume the valences of the chemical elements—the number of electrons with. Why Doesn't Bromine Have 17 Valence Electrons.
From ar.inspiredpencil.com
Bromine Valence Electrons Why Doesn't Bromine Have 17 Valence Electrons Bromine has seven valence electrons. the total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. The electron configuration of an iron atom is 1s 2 2s 2. It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. It is not the. Why Doesn't Bromine Have 17 Valence Electrons.
From dxooqbere.blob.core.windows.net
Bromine Configuration Of Electrons at Daniel Wright blog Why Doesn't Bromine Have 17 Valence Electrons Bromine has an electron configuration of. bromine (br) is a halogen and has an atomic number of 35. the total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. the valence electrons are be the 4s and 4p electrons. the electron configuration. Why Doesn't Bromine Have 17 Valence Electrons.
From brainly.in
bromine valence electrons, Brainly.in Why Doesn't Bromine Have 17 Valence Electrons It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an element. bromine (br) is a halogen and has an atomic number of 35. Therefore, the valence electrons of. the total number of electrons present in the valence shell of an atom are called valence electrons, and there are. Why Doesn't Bromine Have 17 Valence Electrons.
From srkrkzbhxtdlh.blogspot.com
How Many Valence Electrons Does Bromine Have Bromine is a group viia Why Doesn't Bromine Have 17 Valence Electrons you may assume the valences of the chemical elements—the number of electrons with which an atom. the valence electrons are be the 4s and 4p electrons. The electron configuration of an iron atom is 1s 2 2s 2. It is not the valence electrons themselves, but the number of valence electrons that determines the chemical properties of an. Why Doesn't Bromine Have 17 Valence Electrons.