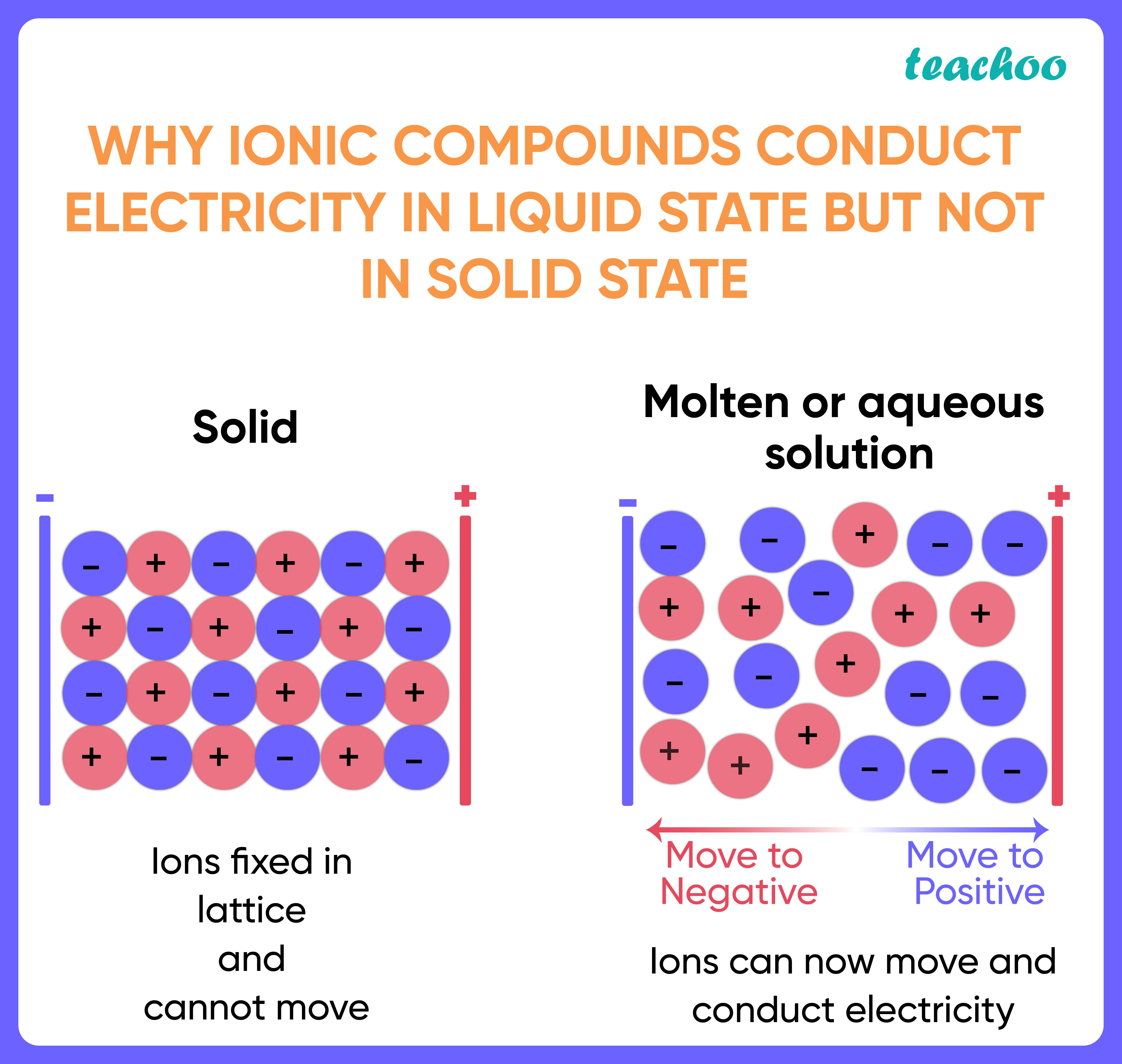
Why Are Ionic Compounds Not Soluble In Water . When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. The solubility of ionic solids in water depends of two things: Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. The solubility of ionic compounds is one of their most basic physical properties. It is understood theoretically that ionic crystals in a. Water molecules in front of and behind the ions are not shown. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. However, to predict whether the compound will dissolve or not, we need to. (1) the energy change, δhdissolve, that occurs when the ionic. When ionic compounds dissolve in water, the ions in the. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. Not all ionic compounds are able. Our article on solubility explains how ionic compounds dissolve in water to form a solution.
from www.myxxgirl.com
(1) the energy change, δhdissolve, that occurs when the ionic. Not all ionic compounds are able. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. Our article on solubility explains how ionic compounds dissolve in water to form a solution. However, to predict whether the compound will dissolve or not, we need to. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. It is understood theoretically that ionic crystals in a. When ionic compounds dissolve in water, the ions in the. The solubility of ionic compounds is one of their most basic physical properties.
Why Do Ionic Compounds Conduct Electricity In Water Sciencing My XXX
Why Are Ionic Compounds Not Soluble In Water The solubility of ionic compounds is one of their most basic physical properties. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Our article on solubility explains how ionic compounds dissolve in water to form a solution. However, to predict whether the compound will dissolve or not, we need to. Water molecules in front of and behind the ions are not shown. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. The solubility of ionic compounds is one of their most basic physical properties. Not all ionic compounds are able. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. The solubility of ionic solids in water depends of two things: Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. When ionic compounds dissolve in water, the ions in the. It is understood theoretically that ionic crystals in a. (1) the energy change, δhdissolve, that occurs when the ionic.
From www.slideserve.com
PPT Ionic and Covalent bonding PowerPoint Presentation, free download Why Are Ionic Compounds Not Soluble In Water The solubility of ionic compounds is one of their most basic physical properties. However, to predict whether the compound will dissolve or not, we need to. (1) the energy change, δhdissolve, that occurs when the ionic. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break. Why Are Ionic Compounds Not Soluble In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 Why Are Ionic Compounds Not Soluble In Water Our article on solubility explains how ionic compounds dissolve in water to form a solution. It is understood theoretically that ionic crystals in a. However, to predict whether the compound will dissolve or not, we need to. When ionic compounds dissolve in water, the ions in the. The solubility of ionic compounds is one of their most basic physical properties.. Why Are Ionic Compounds Not Soluble In Water.
From ecampusontario.pressbooks.pub
11.2 Electrolytes Chemistry Why Are Ionic Compounds Not Soluble In Water The solubility of ionic compounds is one of their most basic physical properties. Water molecules in front of and behind the ions are not shown. (1) the energy change, δhdissolve, that occurs when the ionic. Our article on solubility explains how ionic compounds dissolve in water to form a solution. Review solubility rules for common ionic compounds in water, including. Why Are Ionic Compounds Not Soluble In Water.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is Why Are Ionic Compounds Not Soluble In Water Water molecules in front of and behind the ions are not shown. Our article on solubility explains how ionic compounds dissolve in water to form a solution. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Not all ionic compounds are able. (1) the energy change, δhdissolve, that occurs when. Why Are Ionic Compounds Not Soluble In Water.
From general.chemistrysteps.com
Dissociation of Ionic Compounds Chemistry Steps Why Are Ionic Compounds Not Soluble In Water Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Water molecules in front of and behind the ions are not shown. The solubility of ionic compounds is one of their most basic physical properties. However, to predict whether the compound will dissolve or not, we need to. Ionic compounds dissolve. Why Are Ionic Compounds Not Soluble In Water.
From www.vrogue.co
Solved Activity 2 1 Solubility Chart Use The Chart Be vrogue.co Why Are Ionic Compounds Not Soluble In Water The solubility of ionic solids in water depends of two things: Our article on solubility explains how ionic compounds dissolve in water to form a solution. However, to predict whether the compound will dissolve or not, we need to. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. It is. Why Are Ionic Compounds Not Soluble In Water.
From cassiusfersgardner.blogspot.com
All of the Following Compounds Are Soluble in Water Except Why Are Ionic Compounds Not Soluble In Water Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. (1) the energy change, δhdissolve, that occurs when the ionic. Our article on solubility explains how ionic compounds dissolve in water to form a solution. The solubility of ionic compounds is one of their most basic physical properties. However, to predict whether the compound will. Why Are Ionic Compounds Not Soluble In Water.
From quizizz.com
Properties of ionic compounds Chemistry Quizizz Why Are Ionic Compounds Not Soluble In Water Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Not all ionic compounds are able. When ionic compounds dissolve in water, the ions in the. (1) the. Why Are Ionic Compounds Not Soluble In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Why Are Ionic Compounds Not Soluble In Water It is understood theoretically that ionic crystals in a. (1) the energy change, δhdissolve, that occurs when the ionic. When ionic compounds dissolve in water, the ions in the. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Ionic compounds dissolve in water if the energy given off when the. Why Are Ionic Compounds Not Soluble In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Why Are Ionic Compounds Not Soluble In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. When ionic compounds dissolve in water, the ions in the. Our article on solubility explains how ionic compounds dissolve in water to form a solution. (1) the energy change, δhdissolve, that occurs when the ionic. The solubility. Why Are Ionic Compounds Not Soluble In Water.
From www.slideserve.com
PPT Reaction Stoichiometry Mole Method Calculations PowerPoint Why Are Ionic Compounds Not Soluble In Water Water molecules in front of and behind the ions are not shown. However, to predict whether the compound will dissolve or not, we need to. Our article on solubility explains how ionic compounds dissolve in water to form a solution. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. When ionic compounds dissolve in. Why Are Ionic Compounds Not Soluble In Water.
From study.com
Compound Solubility in Water Overview & Examples Lesson Why Are Ionic Compounds Not Soluble In Water The solubility of ionic solids in water depends of two things: Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. (1) the energy change, δhdissolve, that occurs when the ionic. When ionic compounds dissolve in water, the ions in the. Water molecules in front of and behind the ions are not shown. Ionic compounds. Why Are Ionic Compounds Not Soluble In Water.
From www.slideserve.com
PPT Solubility of Ionic Compounds PowerPoint Presentation, free Why Are Ionic Compounds Not Soluble In Water Water molecules in front of and behind the ions are not shown. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. The solubility of ionic compounds is one of their most basic physical properties. However, to predict whether the compound will dissolve or not, we need to. When ionic compounds dissolve in water, the. Why Are Ionic Compounds Not Soluble In Water.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I Why Are Ionic Compounds Not Soluble In Water The solubility of ionic compounds is one of their most basic physical properties. Not all ionic compounds are able. (1) the energy change, δhdissolve, that occurs when the ionic. Our article on solubility explains how ionic compounds dissolve in water to form a solution. Ionic compounds dissolve in water if the energy given off when the ions interact with water. Why Are Ionic Compounds Not Soluble In Water.
From socratic.org
How can you use electrical conductivity to decide if a compound is Why Are Ionic Compounds Not Soluble In Water Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. It is understood theoretically that ionic crystals in a. The solubility of ionic solids in water depends of two things: Water molecules in front of and behind the. Why Are Ionic Compounds Not Soluble In Water.
From www.chegg.com
Solved 3. Why are some ionic compounds soluble in water and Why Are Ionic Compounds Not Soluble In Water When ionic compounds dissolve in water, the ions in the. Not all ionic compounds are able. It is understood theoretically that ionic crystals in a. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. The solubility of ionic solids in water depends of two things: The. Why Are Ionic Compounds Not Soluble In Water.
From www.sliderbase.com
Properties of Water Presentation Biology Why Are Ionic Compounds Not Soluble In Water Not all ionic compounds are able. The solubility of ionic compounds is one of their most basic physical properties. When ionic compounds dissolve in water, the ions in the. Water molecules in front of and behind the ions are not shown. However, to predict whether the compound will dissolve or not, we need to. Solubility is the maximum amount of. Why Are Ionic Compounds Not Soluble In Water.
From socratic.org
Substance A will not dissolve in water. What can be said about Why Are Ionic Compounds Not Soluble In Water When ionic compounds dissolve in water, the ions in the. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Our article on solubility explains how ionic compounds dissolve in water to form a solution. However, to predict whether the compound will dissolve or not, we need. Why Are Ionic Compounds Not Soluble In Water.
From www.numerade.com
SOLVED Predict whether each of the following ionic compounds is Why Are Ionic Compounds Not Soluble In Water Water molecules in front of and behind the ions are not shown. The solubility of ionic compounds is one of their most basic physical properties. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. When ionic compounds dissolve in water, the ions in the. It is understood theoretically that ionic. Why Are Ionic Compounds Not Soluble In Water.
From www.youtube.com
Predicting solubility of ionic compounds in water YouTube Why Are Ionic Compounds Not Soluble In Water Water molecules in front of and behind the ions are not shown. However, to predict whether the compound will dissolve or not, we need to. When ionic compounds dissolve in water, the ions in the. Our article on solubility explains how ionic compounds dissolve in water to form a solution. When ionic compounds dissolve in water, the ions in the. Why Are Ionic Compounds Not Soluble In Water.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube Why Are Ionic Compounds Not Soluble In Water When ionic compounds dissolve in water, the ions in the. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. It is understood theoretically that ionic crystals in. Why Are Ionic Compounds Not Soluble In Water.
From favpng.com
Ionic Compound Sodium Chloride Electrical Conductivity Water, PNG Why Are Ionic Compounds Not Soluble In Water It is understood theoretically that ionic crystals in a. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Not all ionic compounds are able. The solubility of ionic solids in water depends of two things: The solubility of ionic compounds is one of their most basic physical properties. However, to. Why Are Ionic Compounds Not Soluble In Water.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Why Are Ionic Compounds Not Soluble In Water The solubility of ionic compounds is one of their most basic physical properties. It is understood theoretically that ionic crystals in a. When ionic compounds dissolve in water, the ions in the. Our article on solubility explains how ionic compounds dissolve in water to form a solution. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium. Why Are Ionic Compounds Not Soluble In Water.
From socratic.org
Why are metallic compounds insoluble in water? Socratic Why Are Ionic Compounds Not Soluble In Water It is understood theoretically that ionic crystals in a. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. The solubility of ionic compounds is one of their most basic physical properties. When ionic compounds dissolve in water, the ions in the. The solubility of ionic solids. Why Are Ionic Compounds Not Soluble In Water.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Why Are Ionic Compounds Not Soluble In Water The solubility of ionic compounds is one of their most basic physical properties. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. The solubility of ionic solids in water depends of two things: Our article on solubility explains how ionic compounds dissolve in water to form. Why Are Ionic Compounds Not Soluble In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 Why Are Ionic Compounds Not Soluble In Water When ionic compounds dissolve in water, the ions in the. It is understood theoretically that ionic crystals in a. However, to predict whether the compound will dissolve or not, we need to. The solubility of ionic compounds is one of their most basic physical properties. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent.. Why Are Ionic Compounds Not Soluble In Water.
From smallbusinessron.web.fc2.com
do covalent compounds dissolve in water Why Are Ionic Compounds Not Soluble In Water Not all ionic compounds are able. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. However, to predict whether the compound will dissolve or not, we need to. Solubility is the maximum amount of solute that can. Why Are Ionic Compounds Not Soluble In Water.
From www.numerade.com
SOLVEDWhy are the ionic compounds soluble in water? Why Are Ionic Compounds Not Soluble In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. When ionic compounds dissolve in water, the ions in the. The solubility of ionic compounds is one of their most basic physical properties. (1) the energy change, δhdissolve, that occurs when the ionic. The solubility of ionic. Why Are Ionic Compounds Not Soluble In Water.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Why Are Ionic Compounds Not Soluble In Water Water molecules in front of and behind the ions are not shown. However, to predict whether the compound will dissolve or not, we need to. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. When ionic compounds dissolve in water, the ions in the. Review solubility rules for common ionic compounds in water, including. Why Are Ionic Compounds Not Soluble In Water.
From www.numerade.com
SOLVED Predict whether each of the following ionic compounds is Why Are Ionic Compounds Not Soluble In Water When ionic compounds dissolve in water, the ions in the. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. The solubility of ionic solids in water depends of two things: Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the. Why Are Ionic Compounds Not Soluble In Water.
From www.myxxgirl.com
Why Do Ionic Compounds Conduct Electricity In Water Sciencing My XXX Why Are Ionic Compounds Not Soluble In Water The solubility of ionic compounds is one of their most basic physical properties. However, to predict whether the compound will dissolve or not, we need to. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. When ionic compounds dissolve in water, the ions in the. Water molecules in front of and behind the ions. Why Are Ionic Compounds Not Soluble In Water.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Why Are Ionic Compounds Not Soluble In Water When ionic compounds dissolve in water, the ions in the. The solubility of ionic solids in water depends of two things: Not all ionic compounds are able. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. It is understood theoretically that ionic crystals in a. The solubility of ionic compounds is one of their. Why Are Ionic Compounds Not Soluble In Water.
From ar.inspiredpencil.com
Ionic Compounds Chart Why Are Ionic Compounds Not Soluble In Water The solubility of ionic compounds is one of their most basic physical properties. It is understood theoretically that ionic crystals in a. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. (1) the energy change, δhdissolve, that occurs when the ionic. Ionic compounds dissolve in water if the energy given. Why Are Ionic Compounds Not Soluble In Water.
From www.numerade.com
SOLVED Solubility Rulesfor some ionic compounds in water Soluble Ionic Why Are Ionic Compounds Not Soluble In Water When ionic compounds dissolve in water, the ions in the. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. Ionic compounds dissolve in water if the energy given off when the ions interact with water. Why Are Ionic Compounds Not Soluble In Water.
From kunduz.com
[ANSWERED] Which of the following ionic compounds is soluble in water Why Are Ionic Compounds Not Soluble In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Our article on solubility explains how ionic compounds dissolve in water to form a solution. When ionic compounds dissolve in water, the ions in the. Review solubility rules for common ionic compounds in water, including calcium carbonate,. Why Are Ionic Compounds Not Soluble In Water.