Calorimeter Constant Term . Find out how heat capacity, molar heat capacity,. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Learn how to find the heat capacity of a calorimeter using different methods and examples. Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. To do so, the heat is exchanged with a calibrated object. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. A calorimeter constant is the. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy.
from www.chegg.com
The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. A calorimeter constant is the. To do so, the heat is exchanged with a calibrated object. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to find the heat capacity of a calorimeter using different methods and examples. Calorimetry is used to measure amounts of heat transferred to or from a substance. Find out how heat capacity, molar heat capacity,. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius.
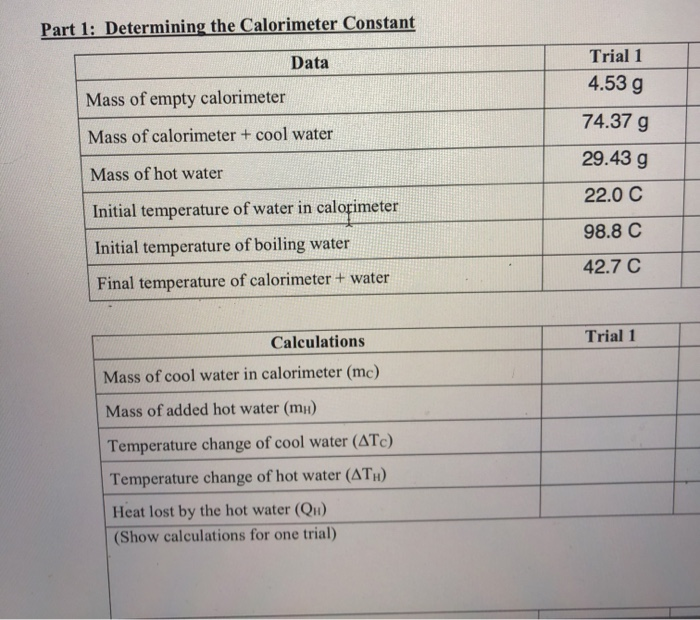
Solved Part 1 Determining the Calorimeter Constant Data
Calorimeter Constant Term A calorimeter constant is the. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Learn how to find the heat capacity of a calorimeter using different methods and examples. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Find out how heat capacity, molar heat capacity,. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. A calorimeter constant is the. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Constant Term Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter constant is the. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. Find out how. Calorimeter Constant Term.
From www.scribd.com
calorimetry Calorimeter Constant Term The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy.. Calorimeter Constant Term.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Constant Term Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to find the heat capacity of a calorimeter using different methods and examples. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. This web page explains the principles and applications of. Calorimeter Constant Term.
From www.pearson.com
Chapter 09 16 Constant Pressure Calorimetry (coffee cup) Channels Calorimeter Constant Term To do so, the heat is exchanged with a calibrated object. A calorimeter constant is the. Calorimetry is used to measure amounts of heat transferred to or from a substance. Find out how heat capacity, molar heat capacity,. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. Learn how to. Calorimeter Constant Term.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimeter Constant Term Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Find out how heat capacity, molar heat capacity,. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. This web page explains the principles and. Calorimeter Constant Term.
From www.indiamart.com
Calorimeter Heat, कैलोरीमीटर in Yash Complex, Vadodara , JP Scientific Calorimeter Constant Term This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. To do so, the heat is exchanged with a calibrated object. Learn how to use calorimetry to measure enthalpy changes in. Calorimeter Constant Term.
From www.youtube.com
How to find calorimeter constant YouTube Calorimeter Constant Term Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimetry is used to measure amounts of heat transferred to or from a substance. Find out how heat capacity, molar heat capacity,. A calorimeter constant is the. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry. Calorimeter Constant Term.
From www.chegg.com
The calorimeter constant value is given in the Calorimeter Constant Term A calorimeter constant is the. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimetry is used to measure amounts of heat transferred to or from a substance. The calorimeter constant is the amount. Calorimeter Constant Term.
From www.scienceabc.com
How Do We Calculate How Many Carbs Are In The Food? Calorimeter Constant Term Learn how to find the heat capacity of a calorimeter using different methods and examples. Find out how heat capacity, molar heat capacity,. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to. Calorimeter Constant Term.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Calorimeter Constant Term A calorimeter constant is the. Calorimetry is used to measure amounts of heat transferred to or from a substance. Find out how heat capacity, molar heat capacity,. To do so, the heat is exchanged with a calibrated object. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. This web. Calorimeter Constant Term.
From www.youtube.com
Chapter 10 16 Constant Pressure Calorimetry (coffee cup) YouTube Calorimeter Constant Term The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. To do so, the heat is exchanged with a calibrated object. A calorimeter constant is the. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. Find out how heat capacity,. Calorimeter Constant Term.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Constant Term Find out how heat capacity, molar heat capacity,. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. Learn how to find the heat capacity of a calorimeter using different methods and examples. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry. Calorimeter Constant Term.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimeter Constant Term To do so, the heat is exchanged with a calibrated object. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. The calorimeter constant, also known as the calorimeter heat capacity, is a. Calorimeter Constant Term.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Calorimeter Constant Term Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Find out how heat capacity, molar heat capacity,. Learn how to find the heat capacity of. Calorimeter Constant Term.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Calorimeter Constant Term The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. Find out how heat capacity, molar heat capacity,. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to use calorimetry to measure. Calorimeter Constant Term.
From www.youtube.com
1A 6.7 ConstantPressure Calorimetry YouTube Calorimeter Constant Term To do so, the heat is exchanged with a calibrated object. Learn how to find the heat capacity of a calorimeter using different methods and examples. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the. Calorimeter Constant Term.
From www.youtube.com
Determining a Calorimeter Constant and specific heat of a metal YouTube Calorimeter Constant Term The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Calorimetry is used to measure amounts of heat transferred to or from a substance. This web page explains the principles and. Calorimeter Constant Term.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Constant Term Learn how to find the heat capacity of a calorimeter using different methods and examples. A calorimeter constant is the. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy.. Calorimeter Constant Term.
From 2012books.lardbucket.org
Calorimetry Calorimeter Constant Term Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. A calorimeter constant is the. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the. Calorimeter Constant Term.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimeter Constant Term Find out how heat capacity, molar heat capacity,. To do so, the heat is exchanged with a calibrated object. A calorimeter constant is the. Learn how to find the heat capacity of a calorimeter using different methods and examples. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. The calorimeter. Calorimeter Constant Term.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Constant Term A calorimeter constant is the. Calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. To do so,. Calorimeter Constant Term.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimeter Constant Term The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. To do so, the heat is exchanged with a calibrated object. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. Learn how to find the heat capacity of. Calorimeter Constant Term.
From www.slideserve.com
PPT Zumdahl Chapter 6 PowerPoint Presentation, free download ID4269919 Calorimeter Constant Term Learn how to find the heat capacity of a calorimeter using different methods and examples. Calorimetry is used to measure amounts of heat transferred to or from a substance. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. To do so, the heat is exchanged with a calibrated object.. Calorimeter Constant Term.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Calorimeter Constant Term Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. A calorimeter constant is the. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the. Calorimeter Constant Term.
From users.highland.edu
Calorimetry Calorimeter Constant Term To do so, the heat is exchanged with a calibrated object. Find out how heat capacity, molar heat capacity,. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. Learn how. Calorimeter Constant Term.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Constant Term This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter constant is the. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. Find. Calorimeter Constant Term.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Constant Term A calorimeter constant is the. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. To do so, the heat is exchanged with a calibrated object. This web page. Calorimeter Constant Term.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Calorimeter Constant Term Find out how heat capacity, molar heat capacity,. A calorimeter constant is the. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. Learn how to write thermochemical equations,. Calorimeter Constant Term.
From jawabanguru.github.io
Parr Adiabatic Bomb Calorimeter Adalah Calorimeter Constant Term This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. A calorimeter constant is the. Learn how to find the heat capacity of a calorimeter using different methods and examples. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to write thermochemical. Calorimeter Constant Term.
From sciencing.com
How to Determine a Calorimeter Constant Sciencing Calorimeter Constant Term The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. Find out how heat capacity, molar heat capacity,. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Learn how to use calorimetry to measure enthalpy changes in chemical processes. Calorimeter Constant Term.
From classnotes.org.in
Measurement Of Change In Internal Energy and Enthalpy Chemistry Calorimeter Constant Term This web page explains the principles and applications of calorimetry, an experimental method to measure heat transfer and enthalpy. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius.. Calorimeter Constant Term.
From www.chegg.com
Solved Part 1 Determining the Calorimeter Constant Data Calorimeter Constant Term Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter constant is the. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to find the heat. Calorimeter Constant Term.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Constant Term Find out how heat capacity, molar heat capacity,. Calorimetry is used to measure amounts of heat transferred to or from a substance. The calorimeter constant is the amount of heat energy required to raise the temperature of the calorimeter by 1 degree celsius. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity. Calorimeter Constant Term.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Calorimeter Constant Term Find out how heat capacity, molar heat capacity,. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. The calorimeter constant, also known as the calorimeter heat capacity, is a measure of the heat capacity of a calorimeter. A calorimeter constant is the. Calorimetry is used to measure amounts of heat. Calorimeter Constant Term.
From studylib.net
Calorimetry Heat of Neutralization Calorimeter Constant Term Find out how heat capacity, molar heat capacity,. Learn how to find the heat capacity of a calorimeter using different methods and examples. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. A calorimeter. Calorimeter Constant Term.