Dors Medical Device . This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. Registration of medical devices with the mhra (the uk. To start with you will need to create an account before you. In the medical devices regulations. For more details, please see our published. This is the introduction on how to use the device online registration system (dors). The mhra public access registration database (pard) website allows you to find: On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration.
from tensentric.com
The mhra public access registration database (pard) website allows you to find: This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. To start with you will need to create an account before you. On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. In the medical devices regulations. This is the introduction on how to use the device online registration system (dors). For more details, please see our published. Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. Registration of medical devices with the mhra (the uk.
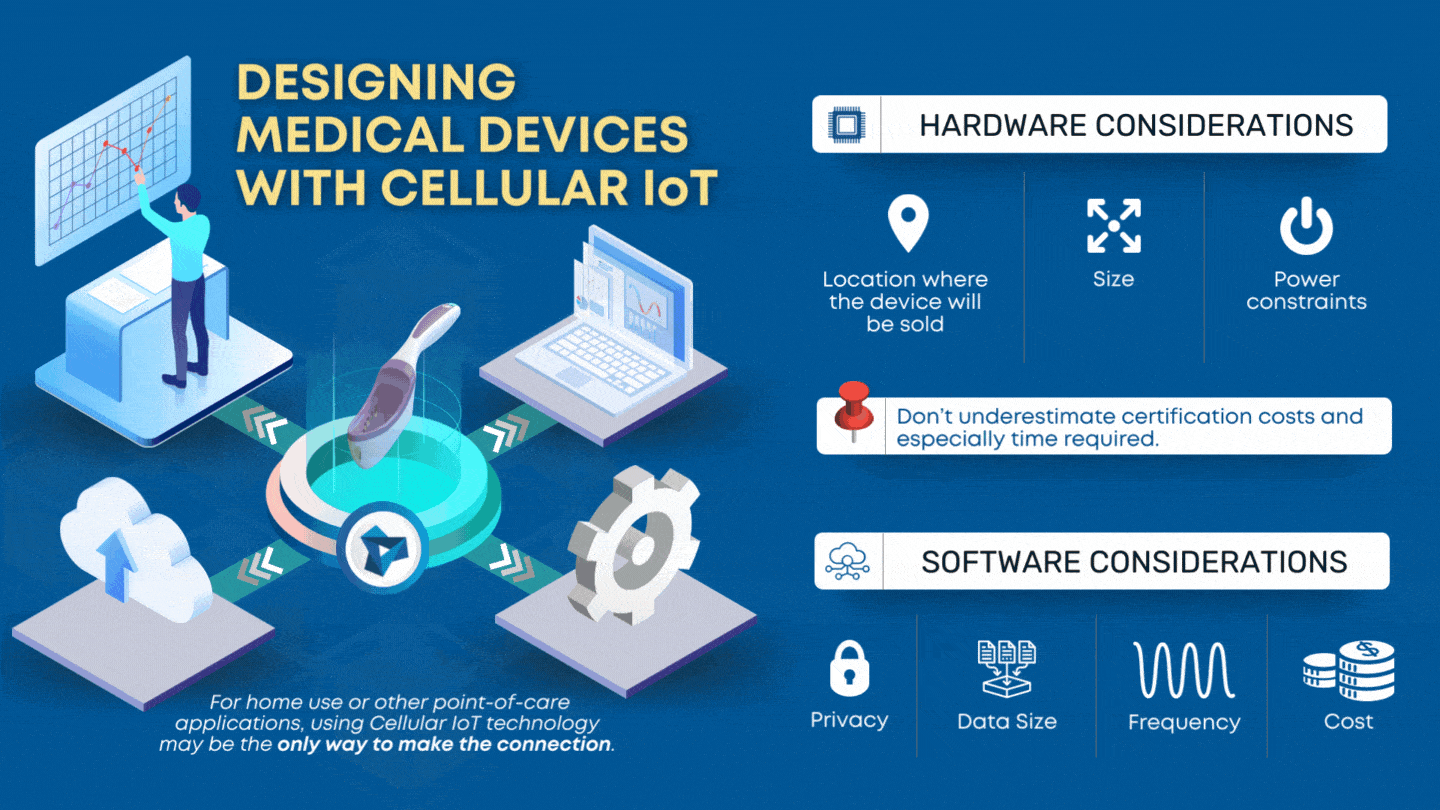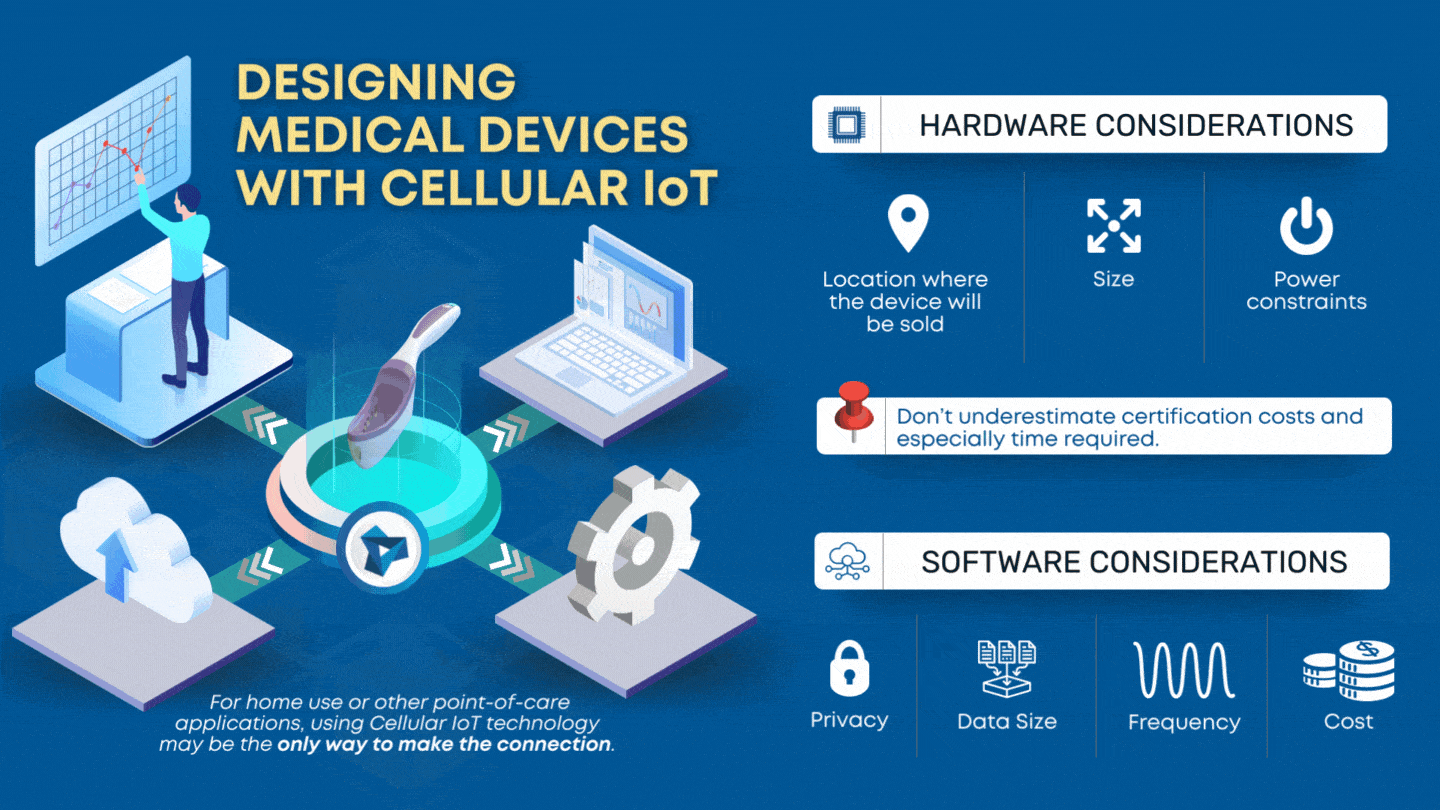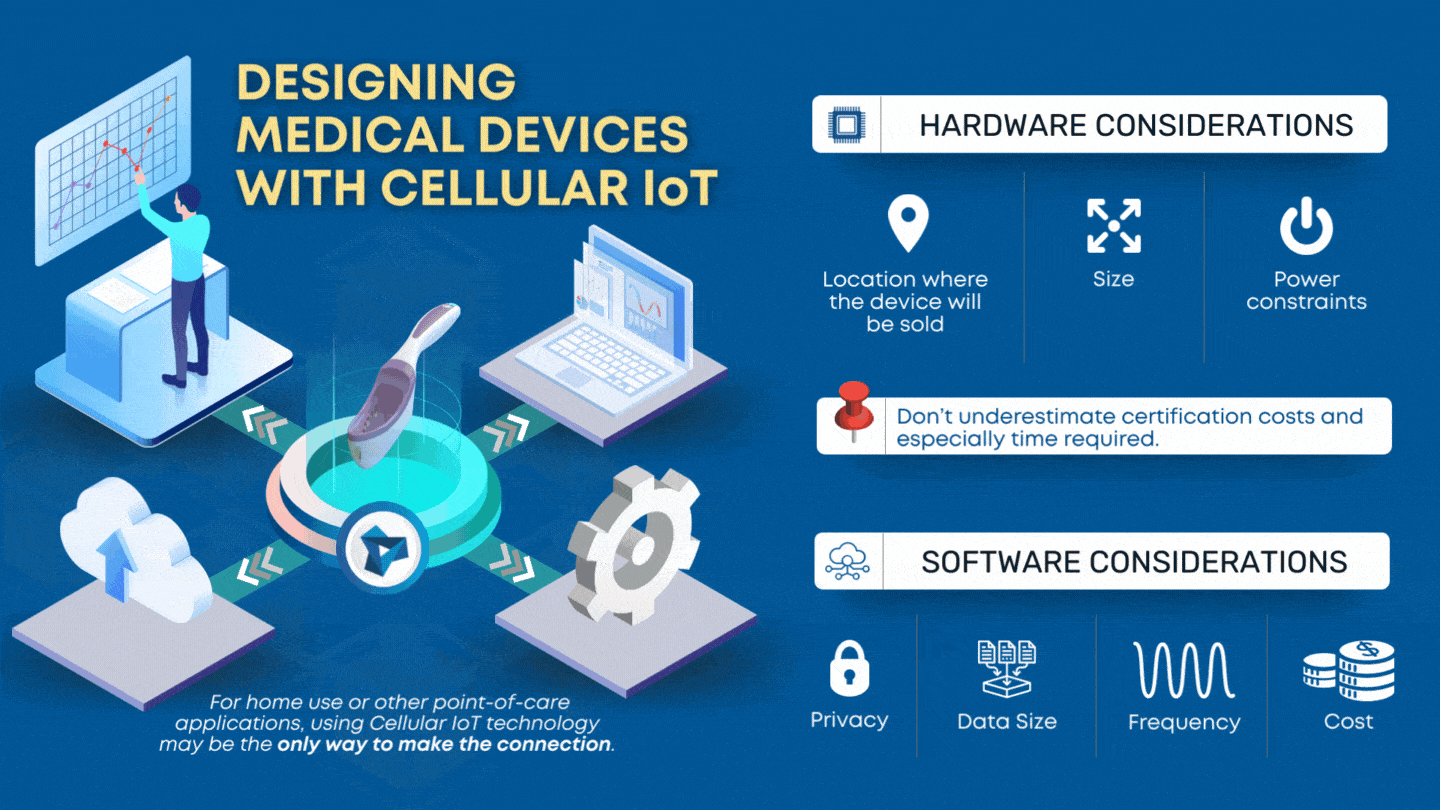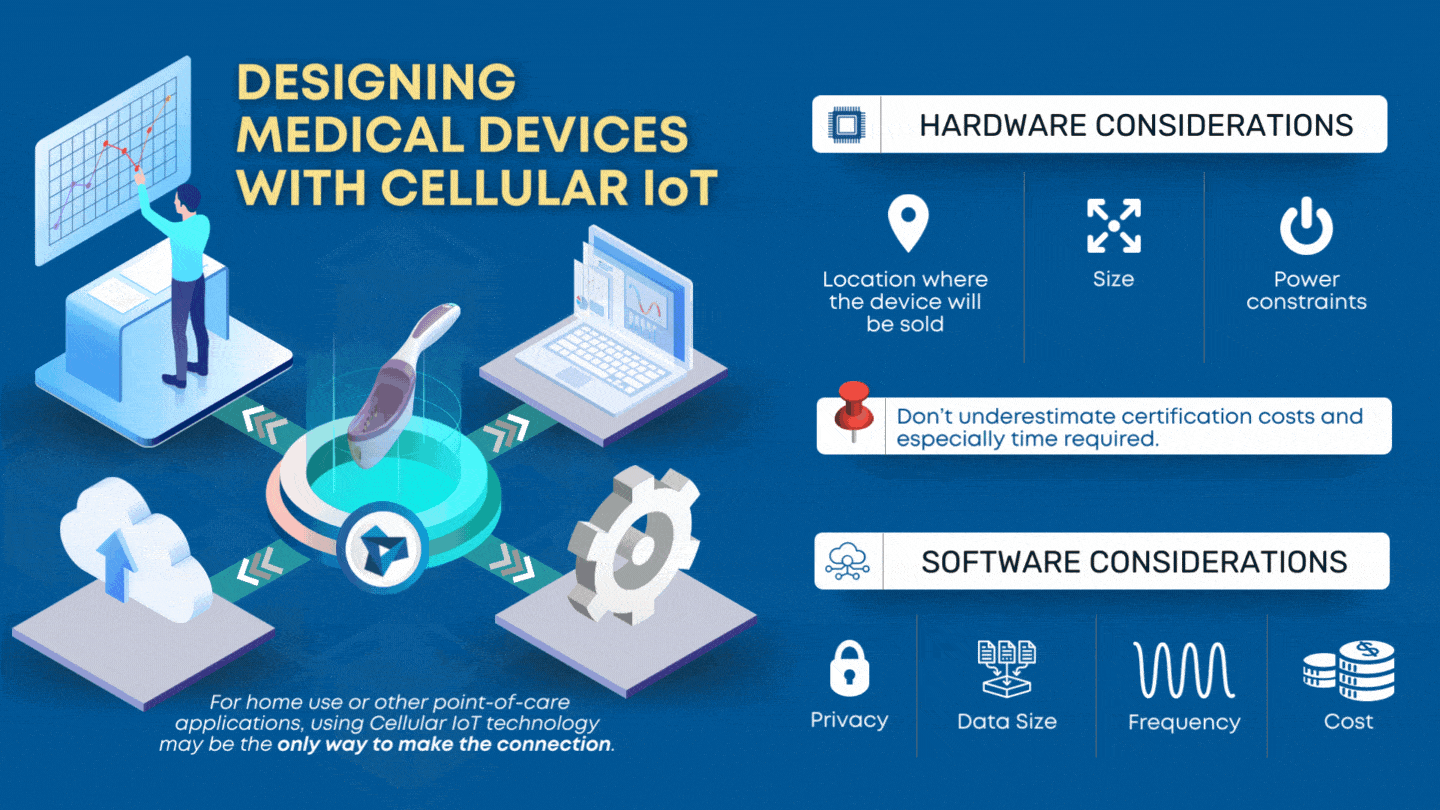
Designing Medical Devices with Cellular IoT Tensentric
Dors Medical Device For more details, please see our published. To start with you will need to create an account before you. Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. The mhra public access registration database (pard) website allows you to find: Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. This is the introduction on how to use the device online registration system (dors). For more details, please see our published. In the medical devices regulations. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. Registration of medical devices with the mhra (the uk. On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration.
From www.iccas.de
DORS Innovation Center Computer Assisted Surgery Dors Medical Device Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. To start with you will need to create an account before you. For more details, please see our published. In the medical devices regulations. This is the introduction on how to use the device online registration system (dors). The mhra public. Dors Medical Device.
From www.btsmedical.gr
Εξεταστικό κρεβάτι DORS BTS Medical Ιατρικός Εξοπλισμός Dors Medical Device This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. For more details, please see our published. On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. To start with you will need. Dors Medical Device.
From royzkala.com
قیمت و خرید دستگاه تشخیص اسکناس مدل DORS 230 باکیفیت بالا در فروشگاه Dors Medical Device This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. In the medical devices regulations. This is the introduction on how to use the device online registration system (dors). Registration of. Dors Medical Device.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk Dors Medical Device In the medical devices regulations. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. The mhra public access registration database (pard) website allows you to find: On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. Regulation (eu) 2023/1545 labelling of. Dors Medical Device.
From www.dornerworks.com
Accelerate Medical Device Development with FPGAs DornerWorks Dors Medical Device Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. Registration of medical devices with the mhra (the uk. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what. Dors Medical Device.
From dors.ro
DORS în Romania prin Ferax Ferax S.R.L. este unicul distribuitor Dors Medical Device This is the introduction on how to use the device online registration system (dors). Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. For more details, please see our published. On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. Registration. Dors Medical Device.
From www.pinterest.de
Take a look at our medical device mounts and find the right product Dors Medical Device Registration of medical devices with the mhra (the uk. In the medical devices regulations. The mhra public access registration database (pard) website allows you to find: Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. This is the introduction on how to use the device online registration system (dors). Regulation. Dors Medical Device.
From dors.ro
SERIA DORS 50/60 DORS în Romania prin Ferax Dors Medical Device Registration of medical devices with the mhra (the uk. In the medical devices regulations. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the. Dors Medical Device.
From bluegoatcyber.com
How Does Medical Device Cybersecurity Relate to ISO 9001 Blue Goat Cyber Dors Medical Device On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. In the medical devices regulations. This is the introduction on how to use. Dors Medical Device.
From operonstrategist.com
Streamline Ophthalmic Medical Device Registration for Class C and D Dors Medical Device For more details, please see our published. Registration of medical devices with the mhra (the uk. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. To start with you will need to create an account before you. On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra). Dors Medical Device.
From bluegoatcyber.com
How Does Medical Device Cybersecurity Relate to ISO 9001 Blue Goat Cyber Dors Medical Device In the medical devices regulations. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. For more details, please see our published. To start with you will need to create an account before you. The mhra public access registration database (pard) website allows you to find: Registration of medical devices with. Dors Medical Device.
From www.avanceforlife.com
DORS For Vitality & General Health Avance Product Dors Medical Device This is the introduction on how to use the device online registration system (dors). To start with you will need to create an account before you. Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. The mhra public access registration database (pard) website allows you to find: On february 11, 2015, the uk's medicines and healthcare products regulatory. Dors Medical Device.
From arapackelaw.com
When Does Software A Medical Device? From SaaS to SaMD Dors Medical Device Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. For more details, please see our published. On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. In the medical devices regulations. This guidance sets out what is required to place a medical device on the gb, ni, and eu. Dors Medical Device.
From www.btsmedical.gr
Εξεταστικό κρεβάτι DORS BTS Medical Ιατρικός Εξοπλισμός Dors Medical Device This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. For more details, please see our published. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. To start with you will need. Dors Medical Device.
From mastermedicalterms.com
dors(o), dors(i) Master Medical Terms Dors Medical Device Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. For more details, please see our published. On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. This guidance sets out. Dors Medical Device.
From dors.ro
DORS 1200 M1 DORS în Romania prin Ferax Dors Medical Device This is the introduction on how to use the device online registration system (dors). Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. In the medical devices regulations. To start with you will need to create an account before. Dors Medical Device.
From www.pdfprof.com
air canada waiver window Dors Medical Device Registration of medical devices with the mhra (the uk. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. To start with you. Dors Medical Device.
From www.youtube.com
Dor procedure Human Heart ️ and Cardiology ️🔊 YouTube Dors Medical Device Registration of medical devices with the mhra (the uk. Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. This is the introduction on how to use the device online registration system (dors). The mhra public access registration database (pard) website allows you to find: For more details, please see our published. Find out more about the conformity assessment. Dors Medical Device.
From www.iccas.de
DORS Innovation Center Computer Assisted Surgery Dors Medical Device The mhra public access registration database (pard) website allows you to find: This is the introduction on how to use the device online registration system (dors). Registration of medical devices with the mhra (the uk. On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. Regulation (eu) 2023/1545 labelling of of. Dors Medical Device.
From www.asphalion.com
Borderline and Classification of medical devices Asphalion Dors Medical Device On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. For more details, please see our published. To start with you will need. Dors Medical Device.
From www.elotouch.com
27” Medical Grade Touchscreen Monitor (Gen 2) Elo® Dors Medical Device This is the introduction on how to use the device online registration system (dors). The mhra public access registration database (pard) website allows you to find: Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does. Dors Medical Device.
From dors.ro
DORS în Romania prin Ferax Ferax S.R.L. este unicul distribuitor Dors Medical Device This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. In the medical devices regulations. Registration of medical devices with the mhra (the uk. For more details, please see our published. Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products.. Dors Medical Device.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Dors Medical Device Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. For more details, please see our published. In the medical devices regulations. The mhra public access registration database (pard) website allows you to find: On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices. Dors Medical Device.
From tensentric.com
Designing Medical Devices with Cellular IoT Tensentric Dors Medical Device In the medical devices regulations. Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. This is the introduction on how to use the device online registration system (dors). On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. This guidance sets out what is required to place a medical. Dors Medical Device.
From dors.ro
DORS în Romania prin Ferax Ferax S.R.L. este unicul distribuitor Dors Medical Device The mhra public access registration database (pard) website allows you to find: On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic. Dors Medical Device.
From www.progressivemedinc.com
What does it take to be a Medical Device Sales Professional? Dors Medical Device This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. In the medical devices regulations. On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. To start with you will need to create. Dors Medical Device.
From dors.maryland.gov
About DORS Dors Medical Device Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. To start with you will need to create an account before you. This is the introduction on how to use the device online registration system (dors). Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. Registration of medical devices. Dors Medical Device.
From www.vseinstrumenti.ru
Универсальный просмотровый детектор DORS 1250 Professional FRZ044867 Dors Medical Device To start with you will need to create an account before you. This is the introduction on how to use the device online registration system (dors). Registration of medical devices with the mhra (the uk. In the medical devices regulations. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. Regulation. Dors Medical Device.
From www.pinterest.com
Pin em Neurology Dors Medical Device Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. This is. Dors Medical Device.
From components.atcgroup.ie
Our Medical Device Product Range Components Dors Medical Device Registration of medical devices with the mhra (the uk. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. To start with you will need to create an account before you. This is the introduction on how to use the device online registration system (dors). On february 11, 2015, the uk's. Dors Medical Device.
From laminarsecurity.com
Medical Device Company Keeps Data Safe With Laminar Dors Medical Device The mhra public access registration database (pard) website allows you to find: For more details, please see our published. In the medical devices regulations. On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. This is the introduction on how to use the device online registration system (dors). This guidance sets. Dors Medical Device.
From www.pinterest.com
Dors Liu on Behance Medical Jokes, Medical Symbols, Medizinisches Dors Medical Device This is the introduction on how to use the device online registration system (dors). Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these. Dors Medical Device.
From dors.ro
DORS în Romania prin Ferax Ferax S.R.L. este unicul distribuitor Dors Medical Device Registration of medical devices with the mhra (the uk. On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. To start with you will need to create an account before you. Regulation (eu) 2023/1545 labelling of of fragrance allergens in cosmetic products. For more details, please see our published. This is. Dors Medical Device.
From www.joharidigital.com
7 Things Medical Device Consultancies must look into while choosing a Dors Medical Device Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on the european. This guidance sets out what is required to place a medical device on the gb, ni, and eu markets, and what mhra does within each of these processes. Registration of medical devices with the mhra (the uk. The mhra public access. Dors Medical Device.
From dors.ro
DORS 1300 M2 DORS în Romania prin Ferax Dors Medical Device The mhra public access registration database (pard) website allows you to find: To start with you will need to create an account before you. On february 11, 2015, the uk's medicines and healthcare products regulatory agency (mhra) will launch the devices online registration. Find out more about the conformity assessment for medical devices in section 2.5 of guidance meddevs on. Dors Medical Device.