Zinc And Copper Sulfate Balanced Equation . Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Balancing step by step using the inspection method. Zn + cu {+2} = zn {+2} + cu. Cupric sulfate + zinc = zinc sulfate + copper. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Let's balance this equation using the.
from www.numerade.com
If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zn + cu {+2} = zn {+2} + cu. Balancing step by step using the inspection method. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction. Let's balance this equation using the. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Cupric sulfate + zinc = zinc sulfate + copper.
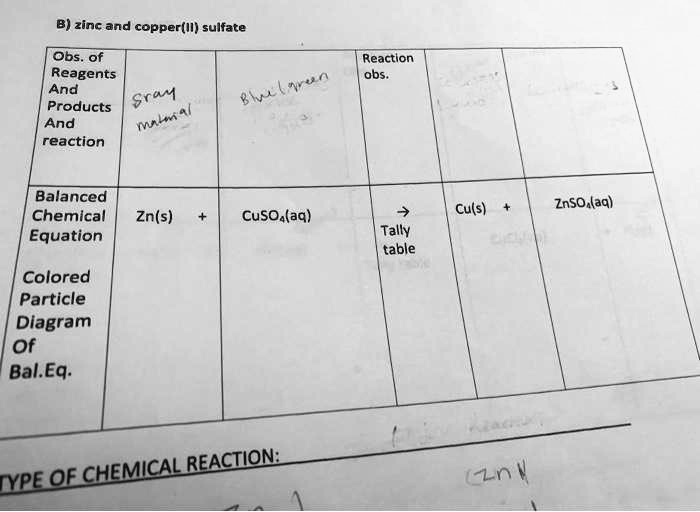
SOLVED B) zinc and copper(II) sulfate Obs Reagents And Srav Products
Zinc And Copper Sulfate Balanced Equation Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zn + cu {+2} = zn {+2} + cu. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Cupric sulfate + zinc = zinc sulfate + copper. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Let's balance this equation using the. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Balancing step by step using the inspection method. Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction.
From www.numerade.com
SOLVED Write the Lewis Dot Structure of Copper Sulfate and Zinc Zinc And Copper Sulfate Balanced Equation An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Let's balance this equation using the. Cuso4. Zinc And Copper Sulfate Balanced Equation.
From www.toppr.com
5. What happens when a] Zinc reacts with Copper Sulphate solution. b Zinc And Copper Sulfate Balanced Equation Cupric sulfate + zinc = zinc sulfate + copper. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. If left in the solution for a longer period. Zinc And Copper Sulfate Balanced Equation.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Zinc And Copper Sulfate Balanced Equation Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Cupric sulfate + zinc = zinc sulfate + copper. Zn + cu {+2} = zn {+2} + cu. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Let's balance this equation using the. Zn + cuso4 = cu2 + znso4 is a single. Zinc And Copper Sulfate Balanced Equation.
From www.youtube.com
How to Balance Zn + CuSO4 = Cu + ZnSO4 (Zinc plus Copper (II) Sulfate Zinc And Copper Sulfate Balanced Equation When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Zn + cu {+2} = zn {+2} + cu. If left in the solution for a longer period of time,. Zinc And Copper Sulfate Balanced Equation.
From www.youtube.com
H2SO4+Zn=ZnSO4+H2 Balanced EquationSulphuric acid+Zinc=Zinc sulphate Zinc And Copper Sulfate Balanced Equation Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). When a strip of zinc metal is placed into. Zinc And Copper Sulfate Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equations 1 zinc react with Zinc And Copper Sulfate Balanced Equation Let's balance this equation using the. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Balancing step by step using the inspection method. Zn + cu {+2} = zn {+2} + cu. Zinc + cupric sulfate = dinuclear copper ion. Zinc And Copper Sulfate Balanced Equation.
From www.numerade.com
SOLVED The reaction between zinc and copper sulphate is illustrated Zinc And Copper Sulfate Balanced Equation Zn + cu {+2} = zn {+2} + cu. Balancing step by step using the inspection method. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). If. Zinc And Copper Sulfate Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the singlereplacement oxidation Zinc And Copper Sulfate Balanced Equation Balancing step by step using the inspection method. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. When a strip of zinc metal is placed into a blue solution of copper (ii). Zinc And Copper Sulfate Balanced Equation.
From www.numerade.com
SOLVED B) zinc and copper(II) sulfate Obs Reagents And Srav Products Zinc And Copper Sulfate Balanced Equation Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Balancing step by step using the inspection method. Zn + cu {+2} = zn {+2} + cu. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to. Zinc And Copper Sulfate Balanced Equation.
From www.toppr.com
1. Write the formula and then balance the following equation. Copper Zinc And Copper Sulfate Balanced Equation When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Cupric sulfate + zinc = zinc sulfate +. Zinc And Copper Sulfate Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Fe + CuSO4 = Fe2(SO4)3 + Cu Zinc And Copper Sulfate Balanced Equation Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Let's balance this equation using the. Zn + cu {+2} = zn {+2} + cu. Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction. Zinc metal reacts with copper (ii) sulfate solution (see. Zinc And Copper Sulfate Balanced Equation.
From brainly.in
Zinc dust is added to blue colour copper sulphate solution.(i) Write Zinc And Copper Sulfate Balanced Equation When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Let's balance this equation using the. Balancing step by step using the inspection method. If left in the solution for a longer period of time, the zinc will gradually decay due. Zinc And Copper Sulfate Balanced Equation.
From www.youtube.com
How to Balance H2SO4 + Zn = ZnSO4 + H2 (Sulfuric acid + Zinc) YouTube Zinc And Copper Sulfate Balanced Equation An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Cupric sulfate + zinc = zinc sulfate + copper. Balancing step by step using the inspection method. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the. Zinc And Copper Sulfate Balanced Equation.
From www.numerade.com
Using the activity series (Table 4.5), write balanced chemical Zinc And Copper Sulfate Balanced Equation Let's balance this equation using the. Balancing step by step using the inspection method. Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Zinc metal reacts with copper (ii) sulfate solution (see right) according. Zinc And Copper Sulfate Balanced Equation.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode Zinc And Copper Sulfate Balanced Equation When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Let's balance this equation using the. Balancing step by step using the inspection method. Cupric sulfate + zinc = zinc sulfate + copper. An animation that shows the process at the. Zinc And Copper Sulfate Balanced Equation.
From hxejkrhaj.blob.core.windows.net
Copper 2 Sulfate Zinc Equation at Viola Smith blog Zinc And Copper Sulfate Balanced Equation An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Zn + cu {+2} = zn {+2} + cu. Balancing step by step using the inspection method. If left in the solution for a longer period. Zinc And Copper Sulfate Balanced Equation.
From keplarllp.com
😀 Zn cuso4 net ionic equation. Help writing complete ionic and net Zinc And Copper Sulfate Balanced Equation Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Zn + cu {+2} = zn {+2} + cu. Cupric sulfate + zinc = zinc sulfate + copper. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Let's balance this equation using the. Zinc + cupric sulfate = dinuclear copper ion + zinc. Zinc And Copper Sulfate Balanced Equation.
From www.chegg.com
Solved The reaction between zinc and copper sulphate is Zinc And Copper Sulfate Balanced Equation Let's balance this equation using the. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. When. Zinc And Copper Sulfate Balanced Equation.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Zinc And Copper Sulfate Balanced Equation Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Zn + cu {+2} = zn {+2} + cu. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Let's balance this equation using the. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Cupric sulfate + zinc = zinc sulfate +. Zinc And Copper Sulfate Balanced Equation.
From www.chegg.com
Solved Reactants Observations Zinc +copper(II) sulfate Zinc And Copper Sulfate Balanced Equation When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zn + cu {+2} = zn {+2} + cu. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc + cupric sulfate = dinuclear copper ion + zinc. Zinc And Copper Sulfate Balanced Equation.
From www.slideshare.net
Redox= quiz part 1 with answers Zinc And Copper Sulfate Balanced Equation When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: An animation that shows the process at the particle level for the reaction between zinc and. Zinc And Copper Sulfate Balanced Equation.
From hxejkrhaj.blob.core.windows.net
Copper 2 Sulfate Zinc Equation at Viola Smith blog Zinc And Copper Sulfate Balanced Equation Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction. Zinc metal reacts with copper (ii) sulfate. Zinc And Copper Sulfate Balanced Equation.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0146 Science Zinc And Copper Sulfate Balanced Equation Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Zn + cuso4 = cu2 + znso4 is a single. Zinc And Copper Sulfate Balanced Equation.
From www.toppr.com
Write a balanced equation for the preparation of the following salts.(i Zinc And Copper Sulfate Balanced Equation If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Balancing step by step using the inspection method. Let's balance this equation using the. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zn + cu {+2} =. Zinc And Copper Sulfate Balanced Equation.
From www.slideshare.net
Replacement reactions Zinc And Copper Sulfate Balanced Equation Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. An animation that shows the process at the particle level for the reaction between zinc and. Zinc And Copper Sulfate Balanced Equation.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc And Copper Sulfate Balanced Equation Balancing step by step using the inspection method. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction. If left in the solution for a longer period of time, the. Zinc And Copper Sulfate Balanced Equation.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc And Copper Sulfate Balanced Equation Let's balance this equation using the. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. An animation that shows the process at the particle level for the reaction between zinc. Zinc And Copper Sulfate Balanced Equation.
From slideplayer.com
Chemical Reactions & Equations ppt download Zinc And Copper Sulfate Balanced Equation Let's balance this equation using the. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Cupric sulfate + zinc = zinc sulfate + copper.. Zinc And Copper Sulfate Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equation, including the states, for Zinc And Copper Sulfate Balanced Equation Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zn + cu {+2} =. Zinc And Copper Sulfate Balanced Equation.
From www.coursehero.com
[Solved] link for the experiment https//youtu.be/ILJhI43wVA Part Zinc And Copper Sulfate Balanced Equation Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction. Cupric sulfate + zinc = zinc sulfate + copper. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation:. Zinc And Copper Sulfate Balanced Equation.
From www.numerade.com
SOLVED The reaction between zinc and copper sulphate is illustrated Zinc And Copper Sulfate Balanced Equation Cupric sulfate + zinc = zinc sulfate + copper. Zn + cu {+2} = zn {+2} + cu. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Balancing step by step using the inspection method. If left in the solution. Zinc And Copper Sulfate Balanced Equation.
From www.chegg.com
Solved mical Reactions REPORT Zinc and Copper Sulfate The Zinc And Copper Sulfate Balanced Equation Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). Zn + cu {+2} = zn {+2} + cu. Balancing step by. Zinc And Copper Sulfate Balanced Equation.
From www.meritnation.com
write a word equation for the reaction of Zinc with copper sulphate Zinc And Copper Sulfate Balanced Equation Let's balance this equation using the. Cupric sulfate + zinc = zinc sulfate + copper. Cuso4 + zn = znso4 + cu is a single displacement (substitution) reaction. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Zinc metal reacts with copper (ii) sulfate solution (see right) according to the following equation: If left in the solution for. Zinc And Copper Sulfate Balanced Equation.
From www.coursehero.com
[Solved] For the reaction between zinc and copper(II) sulfate, describe Zinc And Copper Sulfate Balanced Equation Let's balance this equation using the. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Balancing. Zinc And Copper Sulfate Balanced Equation.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Zinc And Copper Sulfate Balanced Equation Let's balance this equation using the. Balancing step by step using the inspection method. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zn (s) + cuso4 (aq) → znso4 (aq) + cu (s). When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as. Zinc And Copper Sulfate Balanced Equation.