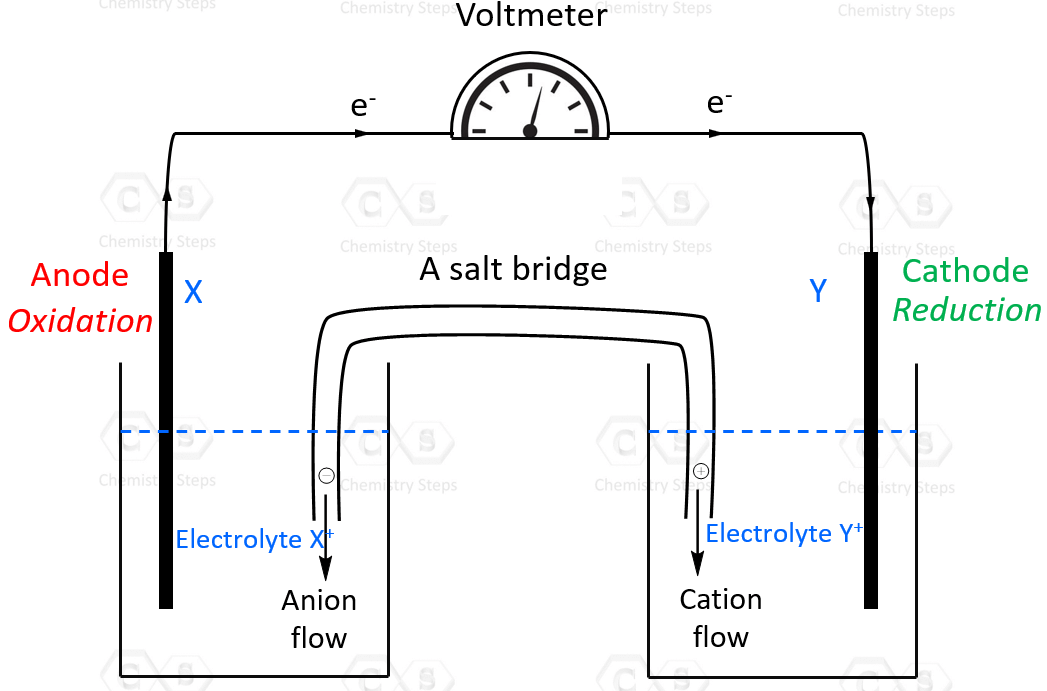
Standard Cell Potential Examples . the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. See examples of cell diagrams and components of an electrochemical cell. See examples of calculating cell potential for redox. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. See examples of concentration cells. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation.
from general.chemistrysteps.com
42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. See examples of concentration cells. See examples of cell diagrams and components of an electrochemical cell. See examples of calculating cell potential for redox. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at.
How to Calculate Standard Cell Potential Chemistry Steps
Standard Cell Potential Examples See examples of cell diagrams and components of an electrochemical cell. See examples of concentration cells. learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. See examples of cell diagrams and components of an electrochemical cell. See examples of calculating cell potential for redox. learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and.
From www.slideserve.com
PPT Lecture 2. Basic Electrochemistry PowerPoint Presentation, free Standard Cell Potential Examples See examples of cell diagrams and components of an electrochemical cell. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. learn how to measure. Standard Cell Potential Examples.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential Examples 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction. Standard Cell Potential Examples.
From www.youtube.com
Chemistry 30 Calculating Standard Cell potential Example YouTube Standard Cell Potential Examples 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. See examples of cell diagrams and components of an electrochemical cell. the potential of the cell under standard conditions (1 m for solutions,. Standard Cell Potential Examples.
From quizdbbarnstorms.z21.web.core.windows.net
What Is Standard Cell Potential Standard Cell Potential Examples learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. See examples of calculating cell potential for redox. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. See examples of cell diagrams and components. Standard Cell Potential Examples.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential Examples the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. See examples of cell diagrams and components of an electrochemical cell. learn the definition. Standard Cell Potential Examples.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Cell Potential Examples learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. See examples of cell diagrams and components of an electrochemical cell. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. See examples of calculating cell potential for redox. the potential of. Standard Cell Potential Examples.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential Examples See examples of cell diagrams and components of an electrochemical cell. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. See examples of calculating cell potential for redox. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. learn how to measure the. Standard Cell Potential Examples.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281515 Standard Cell Potential Examples learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. See examples of concentration cells. learn the definition and formula of standard cell potential, and how to apply. Standard Cell Potential Examples.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Examples See examples of calculating cell potential for redox. See examples of cell diagrams and components of an electrochemical cell. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. See examples of concentration cells. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. . Standard Cell Potential Examples.
From chemwiki.ucdavis.edu
Standard Potentials Chemwiki Standard Cell Potential Examples See examples of cell diagrams and components of an electrochemical cell. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. learn the definition. Standard Cell Potential Examples.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Examples learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. learn how to measure the cell potential (e cell) of an electrochemical cell using. Standard Cell Potential Examples.
From salma-has-blevins.blogspot.com
How to Calculate Cell Potential Under Standard Conditions Salmahas Standard Cell Potential Examples See examples of concentration cells. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. learn how to measure the cell potential (e cell) of an. Standard Cell Potential Examples.
From www.youtube.com
How to Calculate Standard Cell Potential and Voltage Part 1 Examples Standard Cell Potential Examples learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. See examples of cell diagrams and components of an electrochemical cell. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. learn the definition and formula of standard cell potential, and how to. Standard Cell Potential Examples.
From www.youtube.com
Calculating Standard Cell Potential YouTube Standard Cell Potential Examples See examples of calculating cell potential for redox. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. learn the definition and formula of standard cell potential, and how to apply it to. Standard Cell Potential Examples.
From www.youtube.com
How to Calculate Standard Cell Potential and Voltage using E cell = E Standard Cell Potential Examples learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. See examples of calculating cell potential for redox. the potential of the cell under standard conditions (1 m for solutions,. Standard Cell Potential Examples.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential Examples 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. See examples of calculating cell potential for redox. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases,. Standard Cell Potential Examples.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Examples the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. See examples of cell diagrams and components of an electrochemical cell. learn how to measure. Standard Cell Potential Examples.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID6601383 Standard Cell Potential Examples See examples of cell diagrams and components of an electrochemical cell. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. learn how to measure. Standard Cell Potential Examples.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential Examples See examples of cell diagrams and components of an electrochemical cell. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. . Standard Cell Potential Examples.
From www.slideserve.com
PPT ElectroChemistry PowerPoint Presentation, free download ID4652703 Standard Cell Potential Examples learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. See examples of cell diagrams and components of an electrochemical cell. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. See examples of calculating cell potential for redox. . Standard Cell Potential Examples.
From thechemistrynotes.com
Cell Potential & Standard Cell Potential Standard Cell Potential Examples the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. See examples of cell diagrams and components of an electrochemical cell. learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. . Standard Cell Potential Examples.
From www.learntocalculate.com
How to Calculate Cell Potential. Standard Cell Potential Examples learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. See examples of concentration cells. learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. 42 rows calculate the standard cell potential of a voltaic cell that uses the. Standard Cell Potential Examples.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Examples See examples of calculating cell potential for redox. See examples of concentration cells. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. the potential of the cell under. Standard Cell Potential Examples.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Standard Cell Potential Examples the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. See examples of concentration cells. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. See examples of calculating cell potential for redox. learn how. Standard Cell Potential Examples.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281515 Standard Cell Potential Examples See examples of calculating cell potential for redox. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. the potential of the cell under standard conditions (1 m for solutions, 1 atm. Standard Cell Potential Examples.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Standard Cell Potential Examples learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. 42 rows calculate the standard cell potential of a voltaic cell that uses the. Standard Cell Potential Examples.
From www.youtube.com
Standard Cell Potentials YouTube Standard Cell Potential Examples learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. learn how to measure and calculate the reducing power of any element or compound using. Standard Cell Potential Examples.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Cell Potential Examples learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. See examples of cell diagrams and components of an electrochemical cell. See examples of calculating cell potential for redox. learn. Standard Cell Potential Examples.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Cell Potential Examples learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. See examples of calculating cell potential for redox. learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. the potential of the cell under standard conditions (1 m. Standard Cell Potential Examples.
From www.chegg.com
Solved The standard cell potential, E°, for the reaction Standard Cell Potential Examples See examples of concentration cells. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. learn how to measure the cell potential (e cell). Standard Cell Potential Examples.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Standard Cell Potential Examples See examples of calculating cell potential for redox. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. See examples of cell diagrams and components of an electrochemical cell. 42 rows calculate the standard cell potential of a voltaic cell that uses the. Standard Cell Potential Examples.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281515 Standard Cell Potential Examples learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. See examples of calculating cell potential for redox. learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. See examples of cell diagrams and components of an electrochemical cell. learn how to. Standard Cell Potential Examples.
From www.youtube.com
Electrochem 02 Using Standard Cell Potential to Determine Spontaneity Standard Cell Potential Examples learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. . Standard Cell Potential Examples.
From www.bartleby.com
Answered B) Calculate standard cell potential… bartleby Standard Cell Potential Examples See examples of calculating cell potential for redox. learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. See examples of concentration. Standard Cell Potential Examples.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential Examples learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. learn how to measure the cell potential (e cell) of an electrochemical cell using standard reduction potentials and the nernst equation. learn how to measure and calculate the reducing power of any element or compound using standard electrode potential. See. Standard Cell Potential Examples.