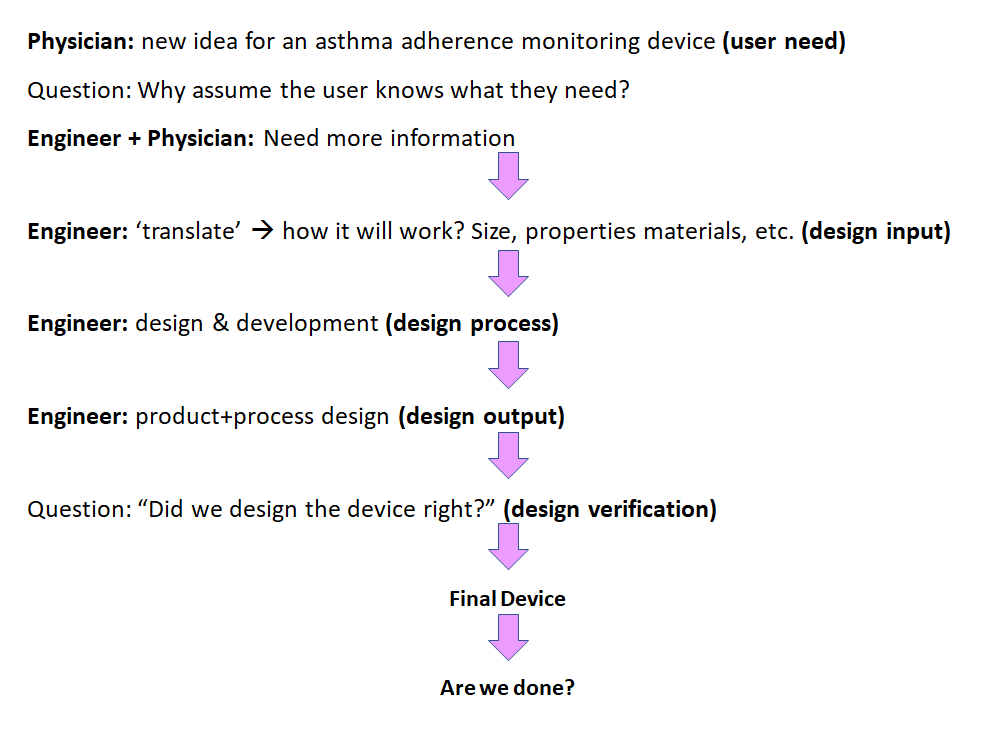
Medical Device Requirements Engineering . How to gather, write, and document product requirements during medical device development to define expected product function, behavior, and performance. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. The advent of integrated, automated lifecycle management tools has helped. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. This white paper explores requirements engineering and its important role in product development and engineering in the medical. Enroll in our online course for medical device requirements engineering. Learn to gather user needs and design input for safe and effective medical devices. Iso 14971 specifies the process for risk management of medical devices, software as a medical device (samd), and in vitro medical devices.
from www.kolabtree.com
The advent of integrated, automated lifecycle management tools has helped. Learn to gather user needs and design input for safe and effective medical devices. This white paper explores requirements engineering and its important role in product development and engineering in the medical. Enroll in our online course for medical device requirements engineering. How to gather, write, and document product requirements during medical device development to define expected product function, behavior, and performance. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. Iso 14971 specifies the process for risk management of medical devices, software as a medical device (samd), and in vitro medical devices. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls.
Medical Device Design The Essential, StepbyStep Guide
Medical Device Requirements Engineering Enroll in our online course for medical device requirements engineering. This white paper explores requirements engineering and its important role in product development and engineering in the medical. Learn to gather user needs and design input for safe and effective medical devices. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. How to gather, write, and document product requirements during medical device development to define expected product function, behavior, and performance. Enroll in our online course for medical device requirements engineering. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. Iso 14971 specifies the process for risk management of medical devices, software as a medical device (samd), and in vitro medical devices. The advent of integrated, automated lifecycle management tools has helped.
From www.credential.net
Medical Device Requirements Engineering • Medical Device HQ Medical Device Requirements Engineering Iso 14971 specifies the process for risk management of medical devices, software as a medical device (samd), and in vitro medical devices. The advent of integrated, automated lifecycle management tools has helped. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. This white paper explores requirements engineering and its important. Medical Device Requirements Engineering.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download Medical Device Requirements Engineering Learn to gather user needs and design input for safe and effective medical devices. Enroll in our online course for medical device requirements engineering. How to gather, write, and document product requirements during medical device development to define expected product function, behavior, and performance. Iso 14971 specifies the process for risk management of medical devices, software as a medical device. Medical Device Requirements Engineering.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Requirements Engineering This white paper explores requirements engineering and its important role in product development and engineering in the medical. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. Iso 14971 specifies the process for risk management of medical devices, software as a medical device (samd), and in vitro. Medical Device Requirements Engineering.
From www.presentationeze.com
Medical Device Regulations. Design Requirements PresentationEZE Medical Device Requirements Engineering The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. Learn to gather user needs and design input for safe and effective medical devices. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. How to gather, write, and. Medical Device Requirements Engineering.
From www.joharidigital.com
Understanding The 7 Phases of Medical Device Development & Manufacturing Medical Device Requirements Engineering This white paper explores requirements engineering and its important role in product development and engineering in the medical. How to gather, write, and document product requirements during medical device development to define expected product function, behavior, and performance. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls.. Medical Device Requirements Engineering.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device Requirements Engineering The advent of integrated, automated lifecycle management tools has helped. This white paper explores requirements engineering and its important role in product development and engineering in the medical. Iso 14971 specifies the process for risk management of medical devices, software as a medical device (samd), and in vitro medical devices. Fda has developed this guidance document to assist industry in. Medical Device Requirements Engineering.
From sterlingmedicaldevices.com
Medical Device Engineering It Takes All Types Medical Device Requirements Engineering Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. How to gather, write, and document product requirements during medical device development to define expected product function, behavior, and performance. The advent of integrated, automated lifecycle management tools has helped. Enroll in our online course for medical device requirements engineering. Learn. Medical Device Requirements Engineering.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Requirements Engineering The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. The advent of integrated, automated lifecycle management tools has helped. This white paper explores requirements engineering and its important. Medical Device Requirements Engineering.
From www.proplate.com
Medical Device Design & Development Electroplating Solutions ProPlate Medical Device Requirements Engineering This white paper explores requirements engineering and its important role in product development and engineering in the medical. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. Iso. Medical Device Requirements Engineering.
From medicaldevices.freyrsolutions.com
UKCA Marking Requirements for Medical Devices Freyr Medical Devices Medical Device Requirements Engineering The advent of integrated, automated lifecycle management tools has helped. How to gather, write, and document product requirements during medical device development to define expected product function, behavior, and performance. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. Learn to gather user needs and design input for safe and. Medical Device Requirements Engineering.
From www.powersystemsdesign.com
Enhancing medicalproduct usability with IEC 62366 Medical Device Requirements Engineering Enroll in our online course for medical device requirements engineering. Learn to gather user needs and design input for safe and effective medical devices. This white paper explores requirements engineering and its important role in product development and engineering in the medical. Iso 14971 specifies the process for risk management of medical devices, software as a medical device (samd), and. Medical Device Requirements Engineering.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Requirements Engineering Learn to gather user needs and design input for safe and effective medical devices. Enroll in our online course for medical device requirements engineering. This white paper explores requirements engineering and its important role in product development and engineering in the medical. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes. Medical Device Requirements Engineering.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Medical Device Requirements Engineering How to gather, write, and document product requirements during medical device development to define expected product function, behavior, and performance. The advent of integrated, automated lifecycle management tools has helped. This white paper explores requirements engineering and its important role in product development and engineering in the medical. Fda has developed this guidance document to assist industry in following appropriate. Medical Device Requirements Engineering.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Medical Device Requirements Engineering Learn to gather user needs and design input for safe and effective medical devices. How to gather, write, and document product requirements during medical device development to define expected product function, behavior, and performance. This white paper explores requirements engineering and its important role in product development and engineering in the medical. Fda has developed this guidance document to assist. Medical Device Requirements Engineering.
From www.qualitymeddev.com
IEC 62304 Medical Device Software Overview of the Main Requirements Medical Device Requirements Engineering The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. Learn to gather user needs and design input for safe and effective medical devices. Enroll in our online course for medical device requirements engineering. How to gather, write, and document product requirements during medical device development to define. Medical Device Requirements Engineering.
From www.modernrequirements.com
Facilitate the Medical Device Design Controls Modern Requirements Medical Device Requirements Engineering This white paper explores requirements engineering and its important role in product development and engineering in the medical. Iso 14971 specifies the process for risk management of medical devices, software as a medical device (samd), and in vitro medical devices. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement. Medical Device Requirements Engineering.
From www.slideshare.net
Systems Engineering and Requirements Management in Medical Device Pro… Medical Device Requirements Engineering The advent of integrated, automated lifecycle management tools has helped. Enroll in our online course for medical device requirements engineering. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. Learn to gather user needs and design input for safe and effective medical devices. The standard outlines a process for medical. Medical Device Requirements Engineering.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Medical Device Requirements Engineering Learn to gather user needs and design input for safe and effective medical devices. The advent of integrated, automated lifecycle management tools has helped. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. Iso 14971 specifies the process for risk management of medical devices, software as a. Medical Device Requirements Engineering.
From medicaldevicehq.com
How to integrate proactive safety by design Medical Device HQ Medical Device Requirements Engineering Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. Enroll in our online course for medical device requirements engineering. How to gather, write, and document product requirements during. Medical Device Requirements Engineering.
From medicaldevicehq.com
Introduction to Medical Device Requirements Engineering course Medical Device Requirements Engineering The advent of integrated, automated lifecycle management tools has helped. Enroll in our online course for medical device requirements engineering. Iso 14971 specifies the process for risk management of medical devices, software as a medical device (samd), and in vitro medical devices. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them,. Medical Device Requirements Engineering.
From www.youtube.com
Tutorial Diagnosing Medical Device Requirements for Healthy Projects Medical Device Requirements Engineering The advent of integrated, automated lifecycle management tools has helped. Iso 14971 specifies the process for risk management of medical devices, software as a medical device (samd), and in vitro medical devices. Learn to gather user needs and design input for safe and effective medical devices. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the. Medical Device Requirements Engineering.
From kiwa-website.vercel.app
ISO 13485_2016 Medical Devices Requirements.png Medical Device Requirements Engineering Enroll in our online course for medical device requirements engineering. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. This white paper explores requirements engineering and its important role in product development and engineering in the medical. The advent of integrated, automated lifecycle management tools has helped.. Medical Device Requirements Engineering.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Medical Device Requirements Engineering Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. How to gather, write, and document product requirements during medical device development to define expected product function, behavior, and performance. This white paper explores requirements engineering and its important role in product development and engineering in the medical. Learn to gather. Medical Device Requirements Engineering.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Medical Device Requirements Engineering The advent of integrated, automated lifecycle management tools has helped. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. Enroll in our online course for medical device requirements. Medical Device Requirements Engineering.
From www.perforce.com
Condensed Guide to Medical Device Requirements Management Perforce Medical Device Requirements Engineering Iso 14971 specifies the process for risk management of medical devices, software as a medical device (samd), and in vitro medical devices. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and. Medical Device Requirements Engineering.
From medicaldevicehq.com
Usability engineering and ISO 14971 risk management for medical devices Medical Device Requirements Engineering The advent of integrated, automated lifecycle management tools has helped. Learn to gather user needs and design input for safe and effective medical devices. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. This white paper explores requirements engineering and its important role in product development and. Medical Device Requirements Engineering.
From operonstrategist.com
GMP Certificate for Medical Devices (Standards and Requirements Medical Device Requirements Engineering How to gather, write, and document product requirements during medical device development to define expected product function, behavior, and performance. The advent of integrated, automated lifecycle management tools has helped. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. This white paper explores requirements engineering and its important role in. Medical Device Requirements Engineering.
From medicaldevicehq.com
Introduction to Medical Device Requirements Engineering course Medical Device Requirements Engineering The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. Enroll in our online course for medical device requirements engineering. Learn to gather user needs and design input for safe and effective medical devices. Fda has developed this guidance document to assist industry in following appropriate human factors. Medical Device Requirements Engineering.
From simbex.com
How to Define Product Requirements for Medical Devices Medical Device Requirements Engineering Enroll in our online course for medical device requirements engineering. This white paper explores requirements engineering and its important role in product development and engineering in the medical. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. Iso 14971 specifies the process for risk management of medical devices, software as. Medical Device Requirements Engineering.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Medical Device Requirements Engineering Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. How to gather, write, and document product requirements during medical device development to define expected product function, behavior, and performance. Enroll in our online course for medical device requirements engineering. This white paper explores requirements engineering and its important role in. Medical Device Requirements Engineering.
From www.presentationeze.com
Medical Devices Directive (MDD) 93/42/EEC Explained PresentationEZE Medical Device Requirements Engineering The advent of integrated, automated lifecycle management tools has helped. Learn to gather user needs and design input for safe and effective medical devices. This white paper explores requirements engineering and its important role in product development and engineering in the medical. How to gather, write, and document product requirements during medical device development to define expected product function, behavior,. Medical Device Requirements Engineering.
From simbex.com
How to Define Product Requirements for Medical Devices Medical Device Requirements Engineering This white paper explores requirements engineering and its important role in product development and engineering in the medical. Learn to gather user needs and design input for safe and effective medical devices. Fda has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to. Enroll in our online course for medical device requirements. Medical Device Requirements Engineering.
From www.tuleap.org
IEC 62304 compliance What are the requirements for Medical Device Medical Device Requirements Engineering Learn to gather user needs and design input for safe and effective medical devices. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls. The advent of integrated, automated lifecycle management tools has helped. How to gather, write, and document product requirements during medical device development to define. Medical Device Requirements Engineering.
From creanova.com
Usability Engineering in Medical Devices industry Creanova Medical Device Requirements Engineering The advent of integrated, automated lifecycle management tools has helped. Iso 14971 specifies the process for risk management of medical devices, software as a medical device (samd), and in vitro medical devices. Enroll in our online course for medical device requirements engineering. This white paper explores requirements engineering and its important role in product development and engineering in the medical.. Medical Device Requirements Engineering.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Medical Device Requirements Engineering Learn to gather user needs and design input for safe and effective medical devices. Enroll in our online course for medical device requirements engineering. This white paper explores requirements engineering and its important role in product development and engineering in the medical. Iso 14971 specifies the process for risk management of medical devices, software as a medical device (samd), and. Medical Device Requirements Engineering.