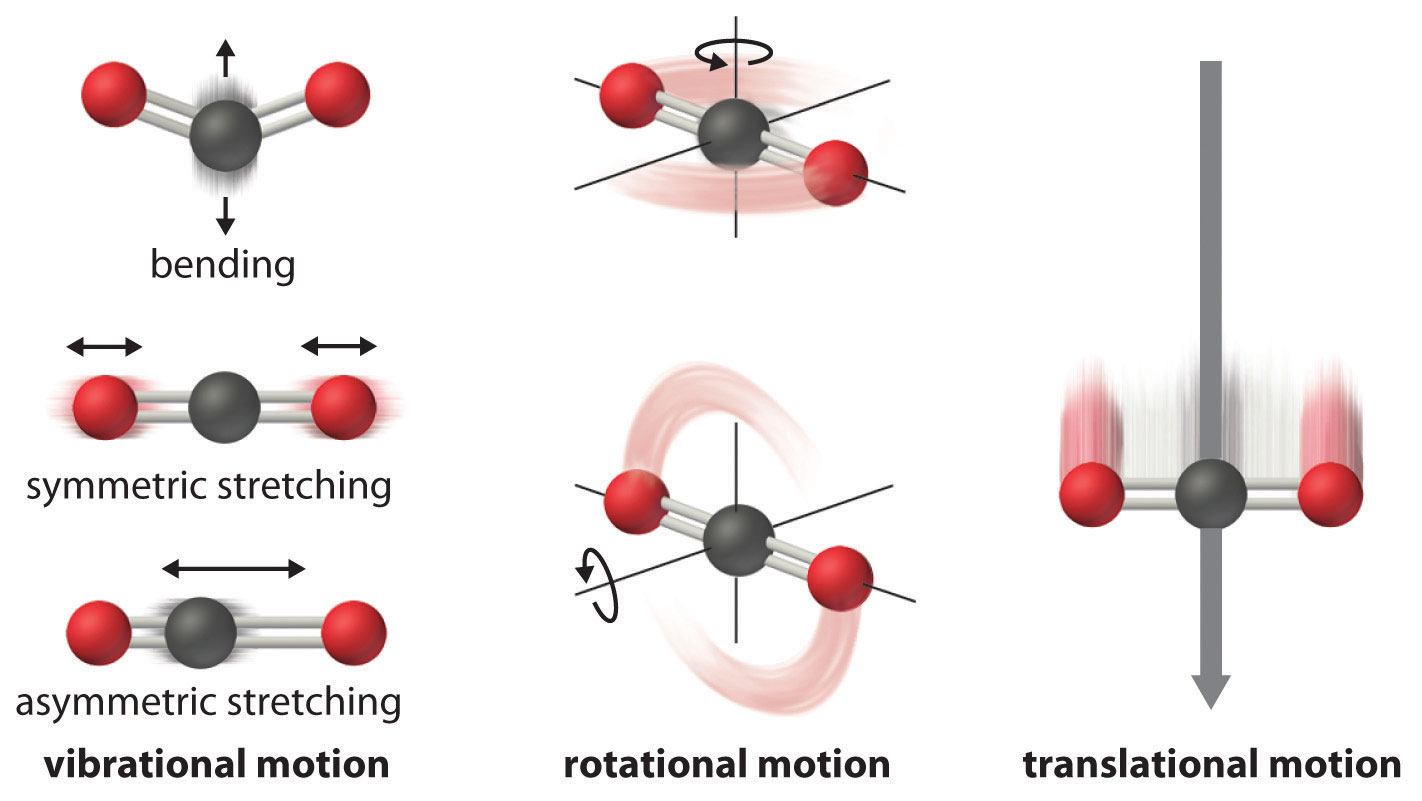
Spectroscopy Chem Libre . This can be analyzed in three ways by measuring. ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most common types of. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. We can use spectroscopy to determine the structure and functional groups. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Spectroscopy is the study of how light interacts with matter. Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). Molecular spectroscopy relates to the interactions that occur between molecules and.
from chem.libretexts.org
This can be analyzed in three ways by measuring. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. We can use spectroscopy to determine the structure and functional groups. Molecular spectroscopy relates to the interactions that occur between molecules and. Spectroscopy is the study of how light interacts with matter. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most common types of. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation).
12 Vibrational Spectroscopy of Diatomic Molecules Chemistry LibreTexts
Spectroscopy Chem Libre Molecular spectroscopy relates to the interactions that occur between molecules and. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most common types of. ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Infrared spectroscopy is the analysis of infrared light interacting with a molecule. This can be analyzed in three ways by measuring. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. We can use spectroscopy to determine the structure and functional groups. Spectroscopy is the study of how light interacts with matter. Molecular spectroscopy relates to the interactions that occur between molecules and. Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation).
From chem.libretexts.org
12.2.1 Colors of Coordination Compounds (Electronic Absorption Spectra Spectroscopy Chem Libre Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. Molecular spectroscopy relates to the interactions that occur between molecules and. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most common types of. Infrared spectroscopy. Spectroscopy Chem Libre.
From www.youtube.com
Gas chromatography Mass spectrometry Instrumentation Advantage Spectroscopy Chem Libre Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. This can be analyzed in three ways by measuring. Spectroscopy is the study of how light interacts with matter. This module is designed to introduce. Spectroscopy Chem Libre.
From www.scribd.com
alloway___ayres_(1987)_chemical_principles_of_environmental_pollution Spectroscopy Chem Libre Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). Molecular spectroscopy relates to the interactions that occur between molecules and. Infrared spectroscopy is the analysis. Spectroscopy Chem Libre.
From www.cannondigi.com
Schematic Diagram Of A Single Beam Uv Vis Spectrophotometer The Best Spectroscopy Chem Libre ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. Spectroscopy is the study of how light interacts. Spectroscopy Chem Libre.
From www.studocu.com
Chemistry Libre Texts ICE Tables ICE Tables An ICE (Initial, Change Spectroscopy Chem Libre ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the. Spectroscopy Chem Libre.
From www.researchgate.net
Espectro Mössbauer de la película de hierro. Download Scientific Diagram Spectroscopy Chem Libre ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. Molecular spectroscopy relates to the interactions that occur. Spectroscopy Chem Libre.
From www.researchgate.net
Schematic diagram of the laserinduced breakdown spectroscopy (LIBS Spectroscopy Chem Libre Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most common types of. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring. Spectroscopy Chem Libre.
From www.youtube.com
Introduction to Spectroscopy .. YouTube Spectroscopy Chem Libre Spectroscopy is the study of how light interacts with matter. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. This can be analyzed in three ways by measuring. ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Most. Spectroscopy Chem Libre.
From store.jenck.com
Espectrofotómetro UVVis UV1900i Jenck store Spectroscopy Chem Libre Molecular spectroscopy relates to the interactions that occur between molecules and. Spectroscopy is the study of how light interacts with matter. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). This module is designed to introduce. Spectroscopy Chem Libre.
From www.researchgate.net
Principle of UVVIS spectrophotometry. (a) Detected UVVIS spectrum for Spectroscopy Chem Libre Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. We can use spectroscopy to determine the structure and functional groups. Molecular spectroscopy relates to the interactions that occur between molecules and. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. This can be. Spectroscopy Chem Libre.
From quizquadratrix.z21.web.core.windows.net
Line Spectrum Of Hydrogen Formula Spectroscopy Chem Libre Infrared spectroscopy is the analysis of infrared light interacting with a molecule. ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. This module is designed to introduce the basic. Spectroscopy Chem Libre.
From pharmagroww.com
Analysis By UV Spectroscopy Methods and Explanations Spectroscopy Chem Libre This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most common types of. We can use spectroscopy to determine the structure and functional groups. Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). ˉv(cm − 1) =. Spectroscopy Chem Libre.
From www.esa.int
ESA Transmission spectroscopy Spectroscopy Chem Libre Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. We can use spectroscopy to determine the structure and functional groups. ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: This can be analyzed in three ways by measuring.. Spectroscopy Chem Libre.
From chem.libretexts.org
4.5 Ultraviolet and visible spectroscopy Chemistry LibreTexts Spectroscopy Chem Libre Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. This can be analyzed in three ways by measuring. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey. Spectroscopy Chem Libre.
From www.technologynetworks.com
Mass Spectrometry Ionization Technology Networks Spectroscopy Chem Libre Molecular spectroscopy relates to the interactions that occur between molecules and. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most common types of. Infrared spectroscopy. Spectroscopy Chem Libre.
From agora.cs.wcu.edu
Chemical Principles Lectures Spectroscopy Chem Libre Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most common types of. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring. Spectroscopy Chem Libre.
From chem.libretexts.org
6.8 ¹³C NMR Spectroscopy Chemistry LibreTexts Spectroscopy Chem Libre Spectroscopy is the study of how light interacts with matter. Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). We can use spectroscopy to determine the structure and functional groups. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several. Spectroscopy Chem Libre.
From florist.buketbunga.com
HP/WA 081214567837 Https Spectroscopy Chem Libre ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the. Spectroscopy Chem Libre.
From www.shutterstock.com
espectrofotometría o espectroscopía utilizadas para las vector de Spectroscopy Chem Libre ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Molecular spectroscopy relates to the interactions that occur between molecules and. Spectroscopy is the study of how light interacts with matter. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam. Spectroscopy Chem Libre.
From dxoeylwqx.blob.core.windows.net
Spectroscopy In Chemistry Pdf at Shawn May blog Spectroscopy Chem Libre Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). Molecular spectroscopy relates to the interactions that occur between molecules and. ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Spectrophotometry is a method to measure how much a chemical substance. Spectroscopy Chem Libre.
From www.smacgigworld.com
Raman Spectroscopy An Outline Spectroscopy Chem Libre Molecular spectroscopy relates to the interactions that occur between molecules and. Spectroscopy is the study of how light interacts with matter. Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several. Spectroscopy Chem Libre.
From www.photonics.com
Wearable Sensor Uses Raman Spectroscopy for Chemical Analysis Spectroscopy Chem Libre Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). Molecular spectroscopy relates to the interactions that occur between molecules and. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the. Spectroscopy Chem Libre.
From pubs.acs.org
Machine Learning for Functional Group Identification in Vibrational Spectroscopy Chem Libre Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Infrared spectroscopy is the analysis of infrared light interacting with a molecule. We can use spectroscopy to determine the structure. Spectroscopy Chem Libre.
From www.compoundchem.com
Analytical Chemistry A Guide to 13C Nuclear Resonance (NMR Spectroscopy Chem Libre Infrared spectroscopy is the analysis of infrared light interacting with a molecule. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most common types of. Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). Molecular spectroscopy relates. Spectroscopy Chem Libre.
From brussels-scientific.com
Chapitre 1 Spectroscopie infrarouge BORZUYA UNIVERSITY Spectroscopy Chem Libre Spectroscopy is the study of how light interacts with matter. ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. We can use spectroscopy to determine the structure and functional. Spectroscopy Chem Libre.
From joiuykrxz.blob.core.windows.net
Ir Spectroscopy Instrumentation Diagram at Roxanne Quiles blog Spectroscopy Chem Libre We can use spectroscopy to determine the structure and functional groups. This can be analyzed in three ways by measuring. Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy:. Spectroscopy Chem Libre.
From chem.libretexts.org
EnergyDispersive Xray Spectroscopy (EDS) Chemistry LibreTexts Spectroscopy Chem Libre We can use spectroscopy to determine the structure and functional groups. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most common types of. ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Molecular spectroscopy relates to the interactions that occur. Spectroscopy Chem Libre.
From chem.libretexts.org
12 Vibrational Spectroscopy of Diatomic Molecules Chemistry LibreTexts Spectroscopy Chem Libre Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: We can use spectroscopy to determine the structure and functional groups. Most of what we know about the structure of. Spectroscopy Chem Libre.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum Spectroscopy Chem Libre Infrared spectroscopy is the analysis of infrared light interacting with a molecule. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most common types of. This can be analyzed in three ways by measuring. Most of what we know about the structure of atoms and molecules comes from studying. Spectroscopy Chem Libre.
From www.istockphoto.com
150+ Spectroscopy Illustrations, graphiques vectoriels libre de droits Spectroscopy Chem Libre ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). Infrared spectroscopy is the analysis of infrared light interacting with a molecule. This module is designed to introduce the basic concepts of. Spectroscopy Chem Libre.
From chem.libretexts.org
4.5 Ultraviolet and visible spectroscopy Chemistry LibreTexts Spectroscopy Chem Libre Infrared spectroscopy is the analysis of infrared light interacting with a molecule. This can be analyzed in three ways by measuring. Spectroscopy is the study of how light interacts with matter. This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most common types of. ˉv(cm − 1) = 1. Spectroscopy Chem Libre.
From www.researchgate.net
Schematic diagram of the laserinduced breakdown spectroscopy (LIBS Spectroscopy Chem Libre Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of. Molecular spectroscopy relates to the interactions that occur between molecules and. ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Spectroscopy is the study of how light interacts with. Spectroscopy Chem Libre.
From es.dreamstime.com
Esquema Del Mecanismo De La Espectrofotometría Ilustración del Vector Spectroscopy Chem Libre This module is designed to introduce the basic concepts of spectroscopy and to provide a survey of several of the most common types of. Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). Spectroscopy is the study of how light interacts with matter. Spectrophotometry is a method to. Spectroscopy Chem Libre.
From chemistrymadesimple.net
How Does A Mass Spectrometer Work? Chemistry Made Simple Spectroscopy Chem Libre ˉv(cm − 1) = 1 λ(μm) × 104(μm cm) = v(hz) c(cm / s) near infrared spectroscopy: Spectroscopy is the study of how light interacts with matter. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. We can use spectroscopy to determine the structure and functional groups. This module is designed to introduce the basic concepts of. Spectroscopy Chem Libre.
From scienceinfo.com
Gas ChromatographyMass Spectrometry (GCMS) Spectroscopy Chem Libre Spectroscopy is the study of how light interacts with matter. Molecular spectroscopy relates to the interactions that occur between molecules and. Infrared spectroscopy is the analysis of infrared light interacting with a molecule. Most of what we know about the structure of atoms and molecules comes from studying their interaction with light (electromagnetic radiation). This can be analyzed in three. Spectroscopy Chem Libre.