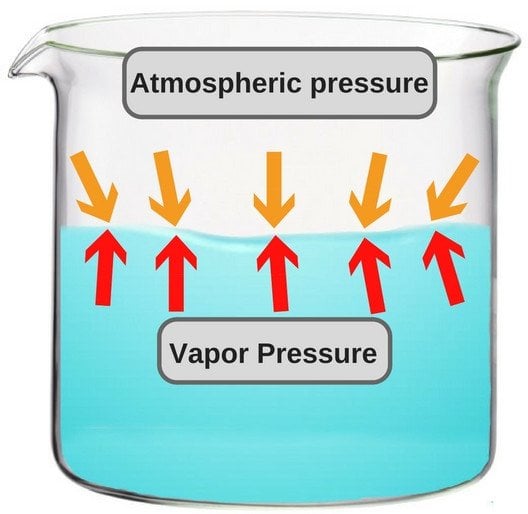
Why Does It Take Longer To Boil Water At Higher Altitudes . Water at sea level boils at 212 degrees fahrenheit; Since this is a naturally. Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. That water takes longer to boil on high. 3,000 feet, when water boils at 206 degrees farenheit,. At 5,000 feet above sea level, the boiling point is 203 degrees f. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). This is the opposite of what many people suppose: Water molecules have an easy time escaping off the surface when the air pressure above them is less. Up at 10,000 feet, water boils at 194 degrees f. For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees farenheit.
from www.scienceabc.com
That water takes longer to boil on high. Since this is a naturally. At 5,000 feet above sea level, the boiling point is 203 degrees f. 3,000 feet, when water boils at 206 degrees farenheit,. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees farenheit. Up at 10,000 feet, water boils at 194 degrees f. Water at sea level boils at 212 degrees fahrenheit; Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. Water molecules have an easy time escaping off the surface when the air pressure above them is less.
Why Does Water Boil Quickly At High Altitudes? » ScienceABC
Why Does It Take Longer To Boil Water At Higher Altitudes At 5,000 feet above sea level, the boiling point is 203 degrees f. Up at 10,000 feet, water boils at 194 degrees f. Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. 3,000 feet, when water boils at 206 degrees farenheit,. Water at sea level boils at 212 degrees fahrenheit; That water takes longer to boil on high. This is the opposite of what many people suppose: Since this is a naturally. At 5,000 feet above sea level, the boiling point is 203 degrees f. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Water molecules have an easy time escaping off the surface when the air pressure above them is less. For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees farenheit.
From cookthink.com
How Long Does it Take for Water to Boil CookThink Why Does It Take Longer To Boil Water At Higher Altitudes This is the opposite of what many people suppose: Water at sea level boils at 212 degrees fahrenheit; Since this is a naturally. That water takes longer to boil on high. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). For instance, at 2,000 feet above sea level, the boiling. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.wonderopolis.org
Why Does Water Boil Faster at Higher Altitude? Wonderopolis Why Does It Take Longer To Boil Water At Higher Altitudes Water molecules have an easy time escaping off the surface when the air pressure above them is less. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Water at sea level boils at 212 degrees fahrenheit; At 5,000 feet above sea level, the boiling point is 203 degrees f. For. Why Does It Take Longer To Boil Water At Higher Altitudes.
From slideplayer.com
WATER And Solution Formation ppt download Why Does It Take Longer To Boil Water At Higher Altitudes One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Water molecules have an easy time escaping off the surface when the air pressure above them is less. For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees farenheit. Water at sea level. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.kindpng.com
Peppa Pig Banned Does Water Boil Faster At Higher Altitudes, HD Png Why Does It Take Longer To Boil Water At Higher Altitudes Water at sea level boils at 212 degrees fahrenheit; Up at 10,000 feet, water boils at 194 degrees f. That water takes longer to boil on high. This is the opposite of what many people suppose: At 5,000 feet above sea level, the boiling point is 203 degrees f. 3,000 feet, when water boils at 206 degrees farenheit,. For instance,. Why Does It Take Longer To Boil Water At Higher Altitudes.
From slideplayer.com
The Extraordinary Properties of Water ppt download Why Does It Take Longer To Boil Water At Higher Altitudes At 5,000 feet above sea level, the boiling point is 203 degrees f. That water takes longer to boil on high. Up at 10,000 feet, water boils at 194 degrees f. 3,000 feet, when water boils at 206 degrees farenheit,. This is the opposite of what many people suppose: For instance, at 2,000 feet above sea level, the boiling point. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.chegg.com
Solved 6) Some Things Take Longer To Cook At High Altitud... Why Does It Take Longer To Boil Water At Higher Altitudes That water takes longer to boil on high. Water molecules have an easy time escaping off the surface when the air pressure above them is less. This is the opposite of what many people suppose: Since this is a naturally. 3,000 feet, when water boils at 206 degrees farenheit,. At 5,000 feet above sea level, the boiling point is 203. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.numerade.com
SOLVEDAt higher altitudes, water boils at lower temperatures. This is Why Does It Take Longer To Boil Water At Higher Altitudes 3,000 feet, when water boils at 206 degrees farenheit,. Water molecules have an easy time escaping off the surface when the air pressure above them is less. Since this is a naturally. Up at 10,000 feet, water boils at 194 degrees f. For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees. Why Does It Take Longer To Boil Water At Higher Altitudes.
From slideplayer.com
The Extraordinary Properties of Water. Water three A water molecule (H Why Does It Take Longer To Boil Water At Higher Altitudes At 5,000 feet above sea level, the boiling point is 203 degrees f. Water at sea level boils at 212 degrees fahrenheit; That water takes longer to boil on high. Since this is a naturally. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). For instance, at 2,000 feet above. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.thespruceeats.com
The Boiling Point of Water at Various Altitudes Why Does It Take Longer To Boil Water At Higher Altitudes Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. Water molecules have an easy time escaping off the surface when the air pressure above them is less. This is the opposite of what many people suppose: Water at sea level boils at 212 degrees fahrenheit; Since this is a naturally. One. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Why Does It Take Longer To Boil Water At Higher Altitudes Since this is a naturally. Water molecules have an easy time escaping off the surface when the air pressure above them is less. Water at sea level boils at 212 degrees fahrenheit; One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Boiling water works as a pathogen reduction method because. Why Does It Take Longer To Boil Water At Higher Altitudes.
From slideplayer.com
The Extraordinary Properties of Water ppt download Why Does It Take Longer To Boil Water At Higher Altitudes Since this is a naturally. Water molecules have an easy time escaping off the surface when the air pressure above them is less. This is the opposite of what many people suppose: That water takes longer to boil on high. Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. 3,000 feet,. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.youtube.com
Does Salt Water Boil Faster? Experiment YouTube Why Does It Take Longer To Boil Water At Higher Altitudes That water takes longer to boil on high. Up at 10,000 feet, water boils at 194 degrees f. At 5,000 feet above sea level, the boiling point is 203 degrees f. Water molecules have an easy time escaping off the surface when the air pressure above them is less. Water at sea level boils at 212 degrees fahrenheit; Since this. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Why Does It Take Longer To Boil Water At Higher Altitudes Since this is a naturally. That water takes longer to boil on high. Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Up at 10,000 feet, water boils at 194 degrees f.. Why Does It Take Longer To Boil Water At Higher Altitudes.
From slideplayer.com
The Extraordinary Properties of Water ppt download Why Does It Take Longer To Boil Water At Higher Altitudes Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. 3,000 feet, when water boils at 206 degrees farenheit,. For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees farenheit. One definition of boiling point is the temperature at which the substance's vapour pressure. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Why Does It Take Longer To Boil Water At Higher Altitudes Up at 10,000 feet, water boils at 194 degrees f. Since this is a naturally. This is the opposite of what many people suppose: One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). 3,000 feet, when water boils at 206 degrees farenheit,. At 5,000 feet above sea level, the boiling. Why Does It Take Longer To Boil Water At Higher Altitudes.
From btechshala.com
How Long Does it Take to Boil Water? Best Time in 2023 Why Does It Take Longer To Boil Water At Higher Altitudes Water molecules have an easy time escaping off the surface when the air pressure above them is less. For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees farenheit. Water at sea level boils at 212 degrees fahrenheit; Since this is a naturally. This is the opposite of what many people suppose:. Why Does It Take Longer To Boil Water At Higher Altitudes.
From giobptqer.blob.core.windows.net
How Long Does It Take Eggs To Boil at Maria Cruz blog Why Does It Take Longer To Boil Water At Higher Altitudes That water takes longer to boil on high. 3,000 feet, when water boils at 206 degrees farenheit,. Water molecules have an easy time escaping off the surface when the air pressure above them is less. Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. For instance, at 2,000 feet above sea. Why Does It Take Longer To Boil Water At Higher Altitudes.
From blog.thermoworks.com
High Altitude and Its Effects on Cooking Why Does It Take Longer To Boil Water At Higher Altitudes Since this is a naturally. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Up at 10,000 feet, water boils at 194 degrees f. Water at sea level boils at 212 degrees fahrenheit; 3,000 feet, when water boils at 206 degrees farenheit,. For instance, at 2,000 feet above sea level,. Why Does It Take Longer To Boil Water At Higher Altitudes.
From brainly.in
Q9. Why does water boil at a lower temperature at higher altitudes Why Does It Take Longer To Boil Water At Higher Altitudes At 5,000 feet above sea level, the boiling point is 203 degrees f. Water at sea level boils at 212 degrees fahrenheit; Water molecules have an easy time escaping off the surface when the air pressure above them is less. Since this is a naturally. Up at 10,000 feet, water boils at 194 degrees f. For instance, at 2,000 feet. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Why Does It Take Longer To Boil Water At Higher Altitudes Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. That water takes longer to boil on high. At 5,000 feet above sea level, the boiling point is 203 degrees f. Water at sea level boils at 212 degrees fahrenheit; This is the opposite of what many people suppose: One definition of. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.gauthmath.com
Solved Which area has atmospheric conditions that produce the lowest Why Does It Take Longer To Boil Water At Higher Altitudes Water at sea level boils at 212 degrees fahrenheit; For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees farenheit. 3,000 feet, when water boils at 206 degrees farenheit,. This is the opposite of what many people suppose: Water molecules have an easy time escaping off the surface when the air pressure. Why Does It Take Longer To Boil Water At Higher Altitudes.
From yesikame.blogspot.com
Temperature Of Boiling Water / Proc Tech & Oper Acad Sensible & Latent Why Does It Take Longer To Boil Water At Higher Altitudes At 5,000 feet above sea level, the boiling point is 203 degrees f. 3,000 feet, when water boils at 206 degrees farenheit,. Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. Since this is a naturally. That water takes longer to boil on high. One definition of boiling point is the. Why Does It Take Longer To Boil Water At Higher Altitudes.
From preprod.bigthink.com
Does water freeze or boil in space? Big Think Why Does It Take Longer To Boil Water At Higher Altitudes At 5,000 feet above sea level, the boiling point is 203 degrees f. Water molecules have an easy time escaping off the surface when the air pressure above them is less. This is the opposite of what many people suppose: Up at 10,000 feet, water boils at 194 degrees f. One definition of boiling point is the temperature at which. Why Does It Take Longer To Boil Water At Higher Altitudes.
From yesikame.blogspot.com
Temperature Of Boiling Water / Proc Tech & Oper Acad Sensible & Latent Why Does It Take Longer To Boil Water At Higher Altitudes That water takes longer to boil on high. Up at 10,000 feet, water boils at 194 degrees f. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees farenheit. This is the opposite. Why Does It Take Longer To Boil Water At Higher Altitudes.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Why Does It Take Longer To Boil Water At Higher Altitudes At 5,000 feet above sea level, the boiling point is 203 degrees f. Since this is a naturally. Water molecules have an easy time escaping off the surface when the air pressure above them is less. Up at 10,000 feet, water boils at 194 degrees f. This is the opposite of what many people suppose: That water takes longer to. Why Does It Take Longer To Boil Water At Higher Altitudes.
From slideplayer.com
The Extraordinary Properties of Water ppt download Why Does It Take Longer To Boil Water At Higher Altitudes Up at 10,000 feet, water boils at 194 degrees f. Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). 3,000 feet, when water boils at 206 degrees farenheit,. Water molecules have an. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.foodchamps.org
How Long Does It Take For Water To Boil? Your Complete Guide Food Champs Why Does It Take Longer To Boil Water At Higher Altitudes This is the opposite of what many people suppose: Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. At 5,000 feet above sea level, the boiling point is 203 degrees f. That water takes longer to boil on high. Up at 10,000 feet, water boils at 194 degrees f. One definition. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country Why Does It Take Longer To Boil Water At Higher Altitudes That water takes longer to boil on high. This is the opposite of what many people suppose: For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees farenheit. Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. At 5,000 feet above sea level,. Why Does It Take Longer To Boil Water At Higher Altitudes.
From hxefdctqz.blob.core.windows.net
How Long To Hard Boil 7 Eggs at Suzanne Salomon blog Why Does It Take Longer To Boil Water At Higher Altitudes 3,000 feet, when water boils at 206 degrees farenheit,. For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees farenheit. Water molecules have an easy time escaping off the surface when the air pressure above them is less. Since this is a naturally. That water takes longer to boil on high. Boiling. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.pinterest.fr
WaterFact Air pressure has an affect on the boiling point of water Why Does It Take Longer To Boil Water At Higher Altitudes Water molecules have an easy time escaping off the surface when the air pressure above them is less. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees farenheit. This is the opposite. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.wave3.com
Behind the Forecast Outwitting the weather when baking Why Does It Take Longer To Boil Water At Higher Altitudes Up at 10,000 feet, water boils at 194 degrees f. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). At 5,000 feet above sea level, the boiling point is 203 degrees f. Since this is a naturally. For instance, at 2,000 feet above sea level, the boiling point of water. Why Does It Take Longer To Boil Water At Higher Altitudes.
From fyonxtdlz.blob.core.windows.net
Does Pure Water Boil At 100 Degrees at Brianna Hunt blog Why Does It Take Longer To Boil Water At Higher Altitudes Water at sea level boils at 212 degrees fahrenheit; Water molecules have an easy time escaping off the surface when the air pressure above them is less. Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. This is the opposite of what many people suppose: Since this is a naturally. At. Why Does It Take Longer To Boil Water At Higher Altitudes.
From hxesyzijm.blob.core.windows.net
How Long Does It Take To Boil Eggs In A Rice Cooker at Kevin Leblanc blog Why Does It Take Longer To Boil Water At Higher Altitudes One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. At 5,000 feet above sea level, the boiling point is 203 degrees f. 3,000 feet, when water boils at 206 degrees farenheit,. Since. Why Does It Take Longer To Boil Water At Higher Altitudes.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Why Does It Take Longer To Boil Water At Higher Altitudes This is the opposite of what many people suppose: For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees farenheit. Boiling water works as a pathogen reduction method because the temperature destroys the essential structures, so to speak,. At 5,000 feet above sea level, the boiling point is 203 degrees f. Up. Why Does It Take Longer To Boil Water At Higher Altitudes.
From beezzly.com
How Long Does It Take to Boil Water? Detailed Guide Beezzly Why Does It Take Longer To Boil Water At Higher Altitudes Up at 10,000 feet, water boils at 194 degrees f. That water takes longer to boil on high. For instance, at 2,000 feet above sea level, the boiling point of water slightly decreases to 208 degrees farenheit. This is the opposite of what many people suppose: One definition of boiling point is the temperature at which the substance's vapour pressure. Why Does It Take Longer To Boil Water At Higher Altitudes.