Table Salt Reaction With Hcl . Table salt / sodium chloride. Sodium chloride is commonly known as table salt. Hydrochloric acid + magnesium →. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. When sodium hydrogen carbonate is treated with. Acid + metal → salt + hydrogen. Baking soda is the common name for sodium hydrogen carbonate (nahco3). Hydrochloric acid + zinc → zinc chloride + hydrogen. When hydrogen chloride gas dissolves in water, (a) it reacts as an acid, transferring protons to water molecules to yield (b) hydronium ions (and solvated chloride ions). Acids react with metals to produce a salt and hydrogen. For instance, ammonia reacts with hydrochloric acid to make ammonium. The hydrogen causes bubbling during the reaction, and can be. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. 2hcl + zn → zncl 2 + h 2. Ammonia forms ammonium salts when it reacts with acids.
from www.youtube.com
For instance, ammonia reacts with hydrochloric acid to make ammonium. 2hcl + zn → zncl 2 + h 2. The hydrogen causes bubbling during the reaction, and can be. Acids react with metals to produce a salt and hydrogen. Table salt / sodium chloride. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Hydrochloric acid + magnesium →. Acid + metal → salt + hydrogen. When hydrogen chloride gas dissolves in water, (a) it reacts as an acid, transferring protons to water molecules to yield (b) hydronium ions (and solvated chloride ions). Hydrochloric acid + zinc → zinc chloride + hydrogen.
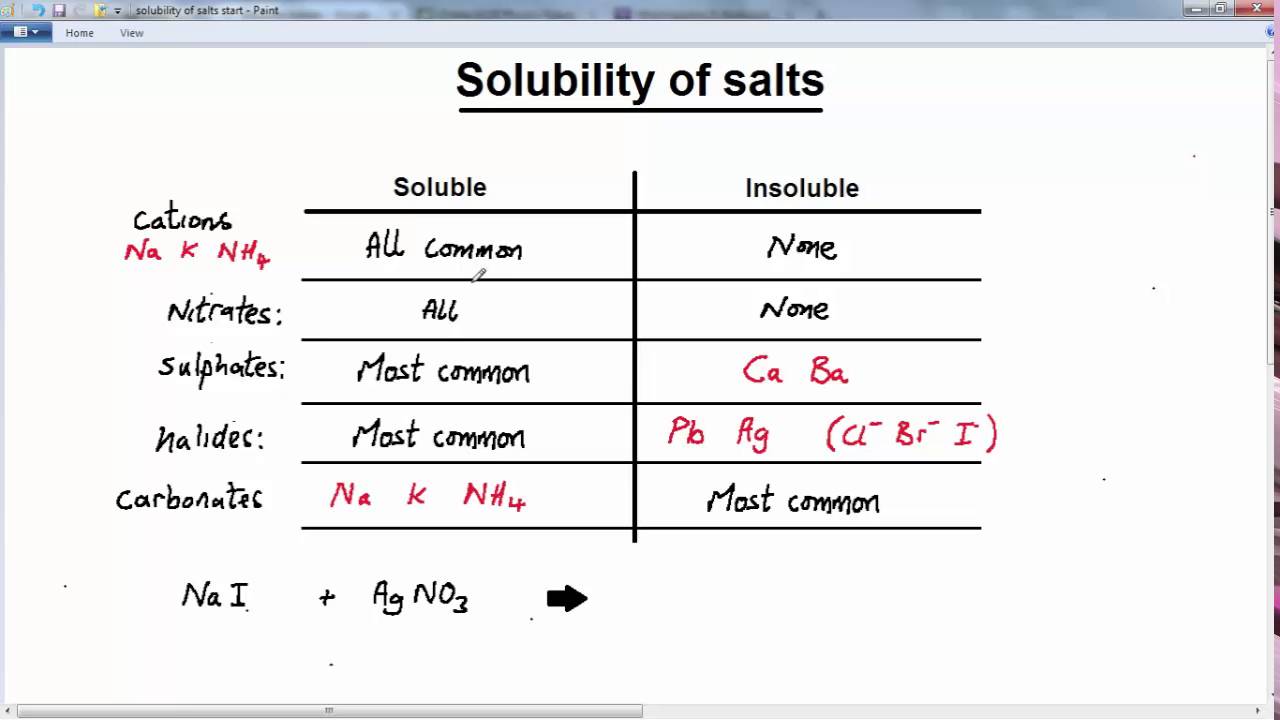
GCSE CHEMISTRY ACIDS AND BASES LESSON 19 salts solubility YouTube
Table Salt Reaction With Hcl Acid + metal → salt + hydrogen. Hydrochloric acid + magnesium →. Acids react with metals to produce a salt and hydrogen. The hydrogen causes bubbling during the reaction, and can be. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. For instance, ammonia reacts with hydrochloric acid to make ammonium. Hydrochloric acid + zinc → zinc chloride + hydrogen. Acid + metal → salt + hydrogen. 2hcl + zn → zncl 2 + h 2. Baking soda is the common name for sodium hydrogen carbonate (nahco3). Ammonia forms ammonium salts when it reacts with acids. When hydrogen chloride gas dissolves in water, (a) it reacts as an acid, transferring protons to water molecules to yield (b) hydronium ions (and solvated chloride ions). When sodium hydrogen carbonate is treated with. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Sodium chloride is commonly known as table salt. Table salt / sodium chloride.
From mammothmemory.net
Sodium reaction with hydrochloric acid is violent and quick Table Salt Reaction With Hcl When sodium hydrogen carbonate is treated with. Baking soda is the common name for sodium hydrogen carbonate (nahco3). When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Table salt / sodium chloride. For instance, ammonia reacts with hydrochloric acid to make ammonium. When hydrogen chloride gas. Table Salt Reaction With Hcl.
From awesomehome.co
Table Salt Chemical Formula Equation Awesome Home Table Salt Reaction With Hcl Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. Hydrochloric acid + zinc → zinc chloride + hydrogen. When sodium hydrogen carbonate is treated with. Sodium chloride is commonly known as table salt. Hydrochloric acid + magnesium →. For instance, ammonia reacts with hydrochloric acid to make ammonium.. Table Salt Reaction With Hcl.
From www.slideshare.net
Metals Table Salt Reaction With Hcl For instance, ammonia reacts with hydrochloric acid to make ammonium. Hydrochloric acid + zinc → zinc chloride + hydrogen. Acids react with metals to produce a salt and hydrogen. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Ammonia forms ammonium salts when it reacts with. Table Salt Reaction With Hcl.
From www.nagwa.com
Question Video Identifying the Sodium Salt That Will Not Produce a Gas Table Salt Reaction With Hcl When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. Acids react with metals to produce a salt and hydrogen. Table salt / sodium chloride. Ammonia. Table Salt Reaction With Hcl.
From www.slideserve.com
PPT ACIDBASE BALANCE PowerPoint Presentation, free download ID6579634 Table Salt Reaction With Hcl Hydrochloric acid + magnesium →. Sodium chloride is commonly known as table salt. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. For instance, ammonia reacts with hydrochloric acid to make ammonium. Hydrochloric acid + zinc → zinc chloride + hydrogen. Baking soda is the common name for. Table Salt Reaction With Hcl.
From fphoto.photoshelter.com
science chemistry neutralization reaction Fundamental Photographs Table Salt Reaction With Hcl Baking soda is the common name for sodium hydrogen carbonate (nahco3). Acids react with metals to produce a salt and hydrogen. Hydrochloric acid + zinc → zinc chloride + hydrogen. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. 2hcl + zn → zncl 2 + h 2.. Table Salt Reaction With Hcl.
From www.chegg.com
Solved 2. Table salt has a molecular formula of NaCl (i.e. 1 Table Salt Reaction With Hcl Sodium chloride is commonly known as table salt. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. The hydrogen causes bubbling during the reaction, and can be. Baking soda is the common name for sodium hydrogen carbonate (nahco3). Acids react with metals to produce a salt and hydrogen.. Table Salt Reaction With Hcl.
From www.youtube.com
Making table salt using sodium metal and chlorine gas YouTube Table Salt Reaction With Hcl Ammonia forms ammonium salts when it reacts with acids. Acids react with metals to produce a salt and hydrogen. Hydrochloric acid + zinc → zinc chloride + hydrogen. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. The hydrogen causes bubbling during the reaction, and can. Table Salt Reaction With Hcl.
From www.alamy.com
Table salt hydrochloric acid and sodium hydroxide Stock Photo 1417560 Table Salt Reaction With Hcl For instance, ammonia reacts with hydrochloric acid to make ammonium. Baking soda is the common name for sodium hydrogen carbonate (nahco3). When sodium hydrogen carbonate is treated with. 2hcl + zn → zncl 2 + h 2. Acid + metal → salt + hydrogen. Hydrochloric acid + zinc → zinc chloride + hydrogen. Acids react with metals to produce a. Table Salt Reaction With Hcl.
From www.teachoo.com
Salts and it's Properties (with Examples) Acids, Bases and Salt Table Salt Reaction With Hcl Table salt / sodium chloride. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. When sodium hydrogen carbonate is treated with. For instance, ammonia reacts with hydrochloric acid to make ammonium. The hydrogen causes bubbling during the reaction, and can be. When hydrogen chloride gas dissolves in water,. Table Salt Reaction With Hcl.
From www.youtube.com
The Reaction Between Hydrochloric Acid and Sodium Carbonate YouTube Table Salt Reaction With Hcl Table salt / sodium chloride. Ammonia forms ammonium salts when it reacts with acids. 2hcl + zn → zncl 2 + h 2. For instance, ammonia reacts with hydrochloric acid to make ammonium. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. Hydrochloric acid + magnesium →. When. Table Salt Reaction With Hcl.
From www.toppr.com
Write equations for the reaction of dil. HCl with each of the following Table Salt Reaction With Hcl Acids react with metals to produce a salt and hydrogen. 2hcl + zn → zncl 2 + h 2. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. Acid + metal → salt + hydrogen. Table salt / sodium chloride. When hydrogen chloride gas dissolves in water, (a). Table Salt Reaction With Hcl.
From stock.adobe.com
Vector illustration of electrolytic dissociation. Molecules break up Table Salt Reaction With Hcl 2hcl + zn → zncl 2 + h 2. The hydrogen causes bubbling during the reaction, and can be. For instance, ammonia reacts with hydrochloric acid to make ammonium. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. When hydrogen chloride gas dissolves in water, (a). Table Salt Reaction With Hcl.
From 88guru.com
What are Salts in Chemistry Characteristics, Types 88guru Table Salt Reaction With Hcl The hydrogen causes bubbling during the reaction, and can be. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. For instance, ammonia reacts with hydrochloric acid to make ammonium. 2hcl + zn → zncl 2 + h 2. Ammonia forms ammonium salts when it reacts with acids. When. Table Salt Reaction With Hcl.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples Table Salt Reaction With Hcl Ammonia forms ammonium salts when it reacts with acids. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. Table salt / sodium chloride. When sodium. Table Salt Reaction With Hcl.
From cabinet.matttroy.net
Table Salt Chemical Formula Matttroy Table Salt Reaction With Hcl When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Hydrochloric acid + zinc → zinc chloride + hydrogen. When sodium hydrogen carbonate is treated with. Sodium chloride is commonly known as table salt. The hydrogen causes bubbling during the reaction, and can be. For instance, ammonia. Table Salt Reaction With Hcl.
From www.slideshare.net
Rate Of Reaction Table Salt Reaction With Hcl Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. When hydrogen chloride gas dissolves in water, (a) it reacts as an acid, transferring protons to water molecules to yield (b) hydronium ions (and solvated chloride ions). Hydrochloric acid + zinc → zinc chloride + hydrogen. Acids react with. Table Salt Reaction With Hcl.
From slideplayer.com
ACIDS & BASES E. Schnobrich. ppt download Table Salt Reaction With Hcl Table salt / sodium chloride. Hydrochloric acid + magnesium →. Hydrochloric acid + zinc → zinc chloride + hydrogen. When hydrogen chloride gas dissolves in water, (a) it reacts as an acid, transferring protons to water molecules to yield (b) hydronium ions (and solvated chloride ions). For instance, ammonia reacts with hydrochloric acid to make ammonium. Baking soda is the. Table Salt Reaction With Hcl.
From www.scribd.com
What Type of Reaction? HCL + Naoh H O + Nacl Neutralization Acid Base Table Salt Reaction With Hcl When sodium hydrogen carbonate is treated with. For instance, ammonia reacts with hydrochloric acid to make ammonium. Sodium chloride is commonly known as table salt. Acid + metal → salt + hydrogen. Table salt / sodium chloride. Hydrochloric acid + zinc → zinc chloride + hydrogen. Acids react with metals to produce a salt and hydrogen. When hydrogen chloride gas. Table Salt Reaction With Hcl.
From elchoroukhost.net
Chemical Equation For Table Salt Elcho Table Table Salt Reaction With Hcl The hydrogen causes bubbling during the reaction, and can be. Acid + metal → salt + hydrogen. Ammonia forms ammonium salts when it reacts with acids. Hydrochloric acid + zinc → zinc chloride + hydrogen. Acids react with metals to produce a salt and hydrogen. 2hcl + zn → zncl 2 + h 2. Sodium chloride is commonly known as. Table Salt Reaction With Hcl.
From www.thesciencehive.co.uk
Chemical tests (GCSE) — the science sauce Table Salt Reaction With Hcl Acid + metal → salt + hydrogen. Baking soda is the common name for sodium hydrogen carbonate (nahco3). 2hcl + zn → zncl 2 + h 2. Sodium chloride is commonly known as table salt. Acids react with metals to produce a salt and hydrogen. Ammonia forms ammonium salts when it reacts with acids. Hydrochloric acid + zinc → zinc. Table Salt Reaction With Hcl.
From stock.adobe.com
Neutralization Reaction Infographic Diagram with example of Table Salt Reaction With Hcl When sodium hydrogen carbonate is treated with. Sodium chloride is commonly known as table salt. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. Acid + metal → salt + hydrogen. Acids react with metals to produce a salt and hydrogen. 2hcl + zn → zncl 2 +. Table Salt Reaction With Hcl.
From courses.lumenlearning.com
4.2 Classifying Chemical Reactions Chemistry Table Salt Reaction With Hcl Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. When hydrogen chloride gas dissolves in water, (a) it reacts as an acid, transferring protons to water molecules to yield (b) hydronium ions (and solvated chloride ions). The hydrogen causes bubbling during the reaction, and can be. When sodium. Table Salt Reaction With Hcl.
From study.com
Neutralization Reaction Definition, Equation & Examples Lesson Table Salt Reaction With Hcl Hydrochloric acid + zinc → zinc chloride + hydrogen. When hydrogen chloride gas dissolves in water, (a) it reacts as an acid, transferring protons to water molecules to yield (b) hydronium ions (and solvated chloride ions). When sodium hydrogen carbonate is treated with. Baking soda is the common name for sodium hydrogen carbonate (nahco3). The hydrogen causes bubbling during the. Table Salt Reaction With Hcl.
From www.youtube.com
GCSE CHEMISTRY ACIDS AND BASES LESSON 19 salts solubility YouTube Table Salt Reaction With Hcl Baking soda is the common name for sodium hydrogen carbonate (nahco3). Acid + metal → salt + hydrogen. Hydrochloric acid + magnesium →. When hydrogen chloride gas dissolves in water, (a) it reacts as an acid, transferring protons to water molecules to yield (b) hydronium ions (and solvated chloride ions). When sodium hydrogen carbonate is treated with. Table salt /. Table Salt Reaction With Hcl.
From www.youtube.com
NaCl + HCl Reaction YouTube Table Salt Reaction With Hcl Sodium chloride is commonly known as table salt. Hydrochloric acid + magnesium →. When hydrogen chloride gas dissolves in water, (a) it reacts as an acid, transferring protons to water molecules to yield (b) hydronium ions (and solvated chloride ions). For instance, ammonia reacts with hydrochloric acid to make ammonium. Table salt / sodium chloride. Sodium chloride (nacl) is commonly. Table Salt Reaction With Hcl.
From www.youtube.com
Identify hydrochloric acid and sulfuric acid solutions HCl and H2SO4 Table Salt Reaction With Hcl For instance, ammonia reacts with hydrochloric acid to make ammonium. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Acids react with metals to produce a salt and hydrogen. Table salt / sodium chloride. When hydrogen chloride gas dissolves in water, (a) it reacts as an. Table Salt Reaction With Hcl.
From www.slideserve.com
PPT How are salts made and named? PowerPoint Presentation, free Table Salt Reaction With Hcl When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Table salt / sodium chloride. When sodium hydrogen carbonate is treated with. Hydrochloric acid + magnesium →. The hydrogen causes bubbling during the reaction, and can be. Ammonia forms ammonium salts when it reacts with acids. Sodium. Table Salt Reaction With Hcl.
From www.coursehero.com
[Solved] Using the solubility curve above, 40 grams of HCl are Table Salt Reaction With Hcl Hydrochloric acid + zinc → zinc chloride + hydrogen. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. For instance, ammonia reacts with hydrochloric acid to make ammonium. Acid + metal → salt + hydrogen. When hydrogen chloride gas dissolves in water, (a) it reacts as an acid,. Table Salt Reaction With Hcl.
From classnotes.ng
Hydrolysis of Salt ClassNotes.ng Table Salt Reaction With Hcl The hydrogen causes bubbling during the reaction, and can be. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. Acids react with metals to produce a salt and hydrogen. Baking soda is the common name for sodium hydrogen carbonate (nahco3). Acid + metal → salt + hydrogen. 2hcl. Table Salt Reaction With Hcl.
From www.numerade.com
SOLVED Solid magnesium reacts with hydrochloric acid (HCI) to form Table Salt Reaction With Hcl Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. The hydrogen causes bubbling during the reaction, and can be. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Hydrochloric acid + magnesium →. Ammonia. Table Salt Reaction With Hcl.
From www.youtube.com
salt hydrolysis and net ionic equations review YouTube Table Salt Reaction With Hcl The hydrogen causes bubbling during the reaction, and can be. Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. Table salt / sodium chloride. Hydrochloric acid + zinc → zinc chloride + hydrogen. Ammonia forms ammonium salts when it reacts with acids. Hydrochloric acid + magnesium →. When. Table Salt Reaction With Hcl.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Table Salt Reaction With Hcl Sodium chloride (nacl) is commonly prepared through the combination of sodium hydroxide (naoh) with hydrochloric acid (hcl) in a neutralization reaction. For instance, ammonia reacts with hydrochloric acid to make ammonium. Acid + metal → salt + hydrogen. Baking soda is the common name for sodium hydrogen carbonate (nahco3). Sodium chloride is commonly known as table salt. 2hcl + zn. Table Salt Reaction With Hcl.
From www.slideserve.com
PPT How are salts made and named? PowerPoint Presentation, free Table Salt Reaction With Hcl The hydrogen causes bubbling during the reaction, and can be. Baking soda is the common name for sodium hydrogen carbonate (nahco3). When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Acids react with metals to produce a salt and hydrogen. When hydrogen chloride gas dissolves in. Table Salt Reaction With Hcl.
From elchoroukhost.net
Chemical Equation For Table Salt And Water Elcho Table Table Salt Reaction With Hcl Hydrochloric acid + zinc → zinc chloride + hydrogen. The hydrogen causes bubbling during the reaction, and can be. Acid + metal → salt + hydrogen. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Hydrochloric acid + magnesium →. Baking soda is the common name. Table Salt Reaction With Hcl.