How Many Energy Levels Are There In Carbon . The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. a measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. as a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36 times. The first energy level can hold up to 2 electrons, and the second can hold up to 8. What must happen for an electron to jump to a different energy. the maximum number of electrons changes dramatically from one energy level to the next. This table shows the pattern in the periodic table that. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. carbon has 2 energy levels. 116 rows electrons orbit the atom's nucleus in energy levels. relate energy levels to the amount of energy their electrons have.
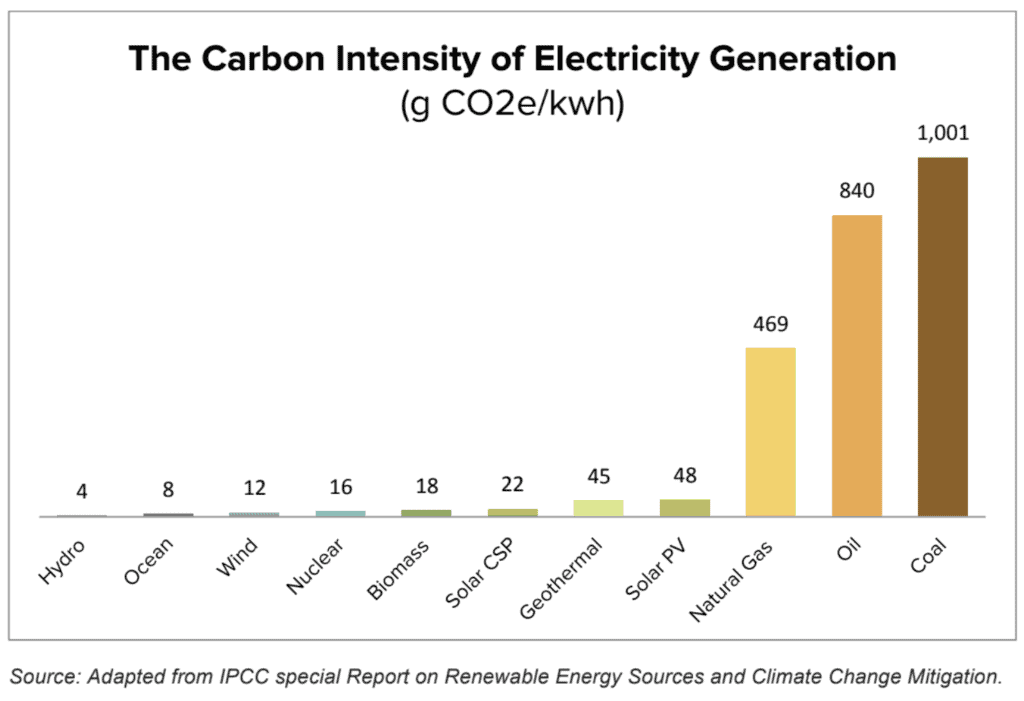
from shrinkthatfootprint.com
This table shows the pattern in the periodic table that. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. What must happen for an electron to jump to a different energy. the maximum number of electrons changes dramatically from one energy level to the next. The first energy level can hold up to 2 electrons, and the second can hold up to 8. a measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. relate energy levels to the amount of energy their electrons have. carbon has 2 energy levels. 116 rows electrons orbit the atom's nucleus in energy levels.
Electricity Emissions Around The World 2023 Shrink That Footprint
How Many Energy Levels Are There In Carbon the maximum number of electrons changes dramatically from one energy level to the next. relate energy levels to the amount of energy their electrons have. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. This table shows the pattern in the periodic table that. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. as a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36 times. What must happen for an electron to jump to a different energy. a measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. 116 rows electrons orbit the atom's nucleus in energy levels. the maximum number of electrons changes dramatically from one energy level to the next. carbon has 2 energy levels. The first energy level can hold up to 2 electrons, and the second can hold up to 8.
From bitcoinmagazine.com
Bitcoin Vs. Financial Sector Energy Use Bitcoin Magazine Bitcoin How Many Energy Levels Are There In Carbon carbon has 2 energy levels. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. relate energy levels to the amount of energy their electrons have. as a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s. How Many Energy Levels Are There In Carbon.
From www.mdpi.com
Materials Free FullText Recent Advances in Carbon NitrideBased S How Many Energy Levels Are There In Carbon carbon has 2 energy levels. the maximum number of electrons changes dramatically from one energy level to the next. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. This table shows the pattern in the periodic table that. 116 rows electrons orbit. How Many Energy Levels Are There In Carbon.
From www.lancaster.ac.uk
XVII Hydrogen atom‣ Quantum Mechanics — Lecture notes for PHYS223 How Many Energy Levels Are There In Carbon The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. relate energy levels to the amount of energy their electrons have. The first energy level can hold up to 2 electrons, and the second can hold up to 8. a measure of how much energy is needed to break all of the bonds of. How Many Energy Levels Are There In Carbon.
From www.slideserve.com
PPT Ch 5 Atomic Structure and the Periodic Table PowerPoint How Many Energy Levels Are There In Carbon the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. relate energy levels to the amount of energy their electrons have. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. carbon has 2 energy levels. a measure of. How Many Energy Levels Are There In Carbon.
From www.weforum.org
Renewable energy is driving new electricity generation in the world’s How Many Energy Levels Are There In Carbon The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. What must happen for an electron to jump to a different energy. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. The first energy level can hold up to 2 electrons,. How Many Energy Levels Are There In Carbon.
From www.tes.com
23 The Carbon Cycle (The Carbon Cycle and Energy Security, Edexcel How Many Energy Levels Are There In Carbon relate energy levels to the amount of energy their electrons have. the maximum number of electrons changes dramatically from one energy level to the next. The first energy level can hold up to 2 electrons, and the second can hold up to 8. What must happen for an electron to jump to a different energy. carbon has. How Many Energy Levels Are There In Carbon.
From sciencing.com
Energy Level Definition, Equation (w/ Diagrams) Sciencing How Many Energy Levels Are There In Carbon a measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. This table shows the pattern in the periodic table that. the maximum number of electrons changes dramatically from one energy level to the next. carbon has 2 energy levels. 116 rows electrons. How Many Energy Levels Are There In Carbon.
From www.sliderbase.com
Electron Quantum Numbers Presentation Chemistry How Many Energy Levels Are There In Carbon The first energy level can hold up to 2 electrons, and the second can hold up to 8. This table shows the pattern in the periodic table that. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. a measure of how much energy is. How Many Energy Levels Are There In Carbon.
From grist.org
After a century of growth, have carbon emissions reached their peak How Many Energy Levels Are There In Carbon The first energy level can hold up to 2 electrons, and the second can hold up to 8. the maximum number of electrons changes dramatically from one energy level to the next. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. as a. How Many Energy Levels Are There In Carbon.
From www.slideserve.com
PPT Ch. 5 “The Periodic Table” PowerPoint Presentation, free download How Many Energy Levels Are There In Carbon carbon has 2 energy levels. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. The first energy level can hold up to 2 electrons, and the second can hold up. How Many Energy Levels Are There In Carbon.
From teroent.com
Climate change Emissions edge up despite drop in coal Tero Enterprises How Many Energy Levels Are There In Carbon carbon has 2 energy levels. What must happen for an electron to jump to a different energy. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the periodic table that. a measure of how much energy. How Many Energy Levels Are There In Carbon.
From www.freeingenergy.com
Solar Emissions Are Far Lower than Other Power Plants Freeing Energy How Many Energy Levels Are There In Carbon 116 rows electrons orbit the atom's nucleus in energy levels. the maximum number of electrons changes dramatically from one energy level to the next. carbon has 2 energy levels. The first energy level can hold up to 2 electrons, and the second can hold up to 8. The ground state electron configuration of carbon is 1s 2. How Many Energy Levels Are There In Carbon.
From sites.uci.edu
Steps to Reducing US CO2 Pollution Energy Blog How Many Energy Levels Are There In Carbon carbon has 2 energy levels. 116 rows electrons orbit the atom's nucleus in energy levels. a measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules,. How Many Energy Levels Are There In Carbon.
From scientifictutor.org
Chem Energy Levels Scientific Tutor How Many Energy Levels Are There In Carbon as a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36 times. 116 rows electrons orbit the atom's nucleus in energy levels. The first energy level can hold up to 2 electrons, and the second can hold up to. How Many Energy Levels Are There In Carbon.
From www.slideserve.com
PPT The fundamental structure of matter ? PowerPoint Presentation How Many Energy Levels Are There In Carbon This table shows the pattern in the periodic table that. 116 rows electrons orbit the atom's nucleus in energy levels. the maximum number of electrons changes dramatically from one energy level to the next. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric.. How Many Energy Levels Are There In Carbon.
From www.vrogue.co
Periodic Table Energy Levels And Sublevels Periodic T vrogue.co How Many Energy Levels Are There In Carbon the maximum number of electrons changes dramatically from one energy level to the next. as a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36 times. What must happen for an electron to jump to a different energy. . How Many Energy Levels Are There In Carbon.
From alternatordiagram.blogspot.com
Energy Level Diagram Chemistry alternator How Many Energy Levels Are There In Carbon relate energy levels to the amount of energy their electrons have. the maximum number of electrons changes dramatically from one energy level to the next. as a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36 times. . How Many Energy Levels Are There In Carbon.
From www.statesmanjournal.com
Climate change Global carbon dioxide emissions reach record high How Many Energy Levels Are There In Carbon a measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. 116 rows electrons orbit the atom's nucleus in energy levels. the maximum number of electrons changes dramatically from one energy level to the next. the term is commonly used for the energy. How Many Energy Levels Are There In Carbon.
From utedzz.blogspot.com
Labeled Periodic Table Energy Levels Periodic Table Timeline How Many Energy Levels Are There In Carbon The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. What must happen for an electron to jump to a different energy. This table shows the pattern in the periodic table that. The first energy level can hold up to 2 electrons, and the second can hold up to 8. 116 rows electrons orbit the. How Many Energy Levels Are There In Carbon.
From shrinkthatfootprint.com
Electricity Emissions Around The World 2023 Shrink That Footprint How Many Energy Levels Are There In Carbon the maximum number of electrons changes dramatically from one energy level to the next. This table shows the pattern in the periodic table that. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. as a result of the z 2 dependence of energy. How Many Energy Levels Are There In Carbon.
From hubpages.com
Atoms and Atomic Structure HubPages How Many Energy Levels Are There In Carbon 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the periodic table that. The first energy level can hold up to 2 electrons, and the second can hold up to 8. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound. How Many Energy Levels Are There In Carbon.
From www.youtube.com
Energy Levels & The Periodic Table YouTube How Many Energy Levels Are There In Carbon The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. The first energy level can hold up to 2 electrons, and the second can hold up to 8. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. 116 rows electrons. How Many Energy Levels Are There In Carbon.
From www.slideserve.com
PPT Properties of Atoms and the Periodic Table PowerPoint How Many Energy Levels Are There In Carbon carbon has 2 energy levels. as a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36 times. This table shows the pattern in the periodic table that. the maximum number of electrons changes dramatically from one energy level. How Many Energy Levels Are There In Carbon.
From www.bbc.co.uk
Climate change and coronavirus Five charts about the biggest carbon How Many Energy Levels Are There In Carbon the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. carbon has 2 energy levels. The first energy level can hold up to 2 electrons, and the second can hold up to 8. relate energy levels to the amount of energy their electrons have.. How Many Energy Levels Are There In Carbon.
From www.weforum.org
Ten charts show how the world is progressing on clean energy World How Many Energy Levels Are There In Carbon 116 rows electrons orbit the atom's nucleus in energy levels. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. What must happen for an electron to jump to a different energy. the maximum number of electrons changes dramatically from one energy level to the next. a measure of how much energy is. How Many Energy Levels Are There In Carbon.
From www.canadianenergycentre.ca
Carbon emissions and coalfired power in Asia Comparisons and new How Many Energy Levels Are There In Carbon The first energy level can hold up to 2 electrons, and the second can hold up to 8. This table shows the pattern in the periodic table that. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. a measure of how much energy is needed to break all of the bonds of the same. How Many Energy Levels Are There In Carbon.
From mavink.com
Periodic Table With Energy Levels How Many Energy Levels Are There In Carbon relate energy levels to the amount of energy their electrons have. the maximum number of electrons changes dramatically from one energy level to the next. This table shows the pattern in the periodic table that. carbon has 2 energy levels. the term is commonly used for the energy levels of the electrons in atoms, ions, or. How Many Energy Levels Are There In Carbon.
From utedzz.blogspot.com
Periodic Table Energy Levels Periodic Table Timeline How Many Energy Levels Are There In Carbon This table shows the pattern in the periodic table that. relate energy levels to the amount of energy their electrons have. the maximum number of electrons changes dramatically from one energy level to the next. The first energy level can hold up to 2 electrons, and the second can hold up to 8. The ground state electron configuration. How Many Energy Levels Are There In Carbon.
From www.cleanenergywire.org
Germany’s greenhouse gas emissions and climate targets Clean Energy Wire How Many Energy Levels Are There In Carbon the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. carbon has 2 energy levels. as a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly. How Many Energy Levels Are There In Carbon.
From climatechange.chicago.gov
Overview of Greenhouse Gases Greenhouse Gas (GHG) Emissions US EPA How Many Energy Levels Are There In Carbon 116 rows electrons orbit the atom's nucleus in energy levels. The first energy level can hold up to 2 electrons, and the second can hold up to 8. This table shows the pattern in the periodic table that. carbon has 2 energy levels. the maximum number of electrons changes dramatically from one energy level to the next.. How Many Energy Levels Are There In Carbon.
From www.alamy.com
A carbon atom, with mass and energy levels. Vector illustration Stock How Many Energy Levels Are There In Carbon as a result of the z 2 dependence of energy in equation 2.24, electrons in the 1s orbital of carbon, which has a nuclear charge of +6, lie roughly 36 times. carbon has 2 energy levels. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by. How Many Energy Levels Are There In Carbon.
From learningschoolequalrf.z22.web.core.windows.net
Explain Bohr's Atomic Model How Many Energy Levels Are There In Carbon The first energy level can hold up to 2 electrons, and the second can hold up to 8. This table shows the pattern in the periodic table that. relate energy levels to the amount of energy their electrons have. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. What must happen for an electron. How Many Energy Levels Are There In Carbon.
From www.slideserve.com
PPT Chapter 71 Electrons and their Energy Levels Part 2 PowerPoint How Many Energy Levels Are There In Carbon 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the periodic table that. the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. a measure of how much energy is needed to break all of the bonds. How Many Energy Levels Are There In Carbon.
From www.slideserve.com
PPT Electron Orbitals PowerPoint Presentation ID2083443 How Many Energy Levels Are There In Carbon This table shows the pattern in the periodic table that. a measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. The first energy level can hold up to 2 electrons, and the second can hold up to 8. relate energy levels to the amount. How Many Energy Levels Are There In Carbon.
From www.stonecoldhands.com
Electron Configurations First Three Rows Stone Cold Chemistry Talk How Many Energy Levels Are There In Carbon the term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric. the maximum number of electrons changes dramatically from one energy level to the next. The first energy level can hold up to 2 electrons, and the second can hold up to 8. carbon has. How Many Energy Levels Are There In Carbon.