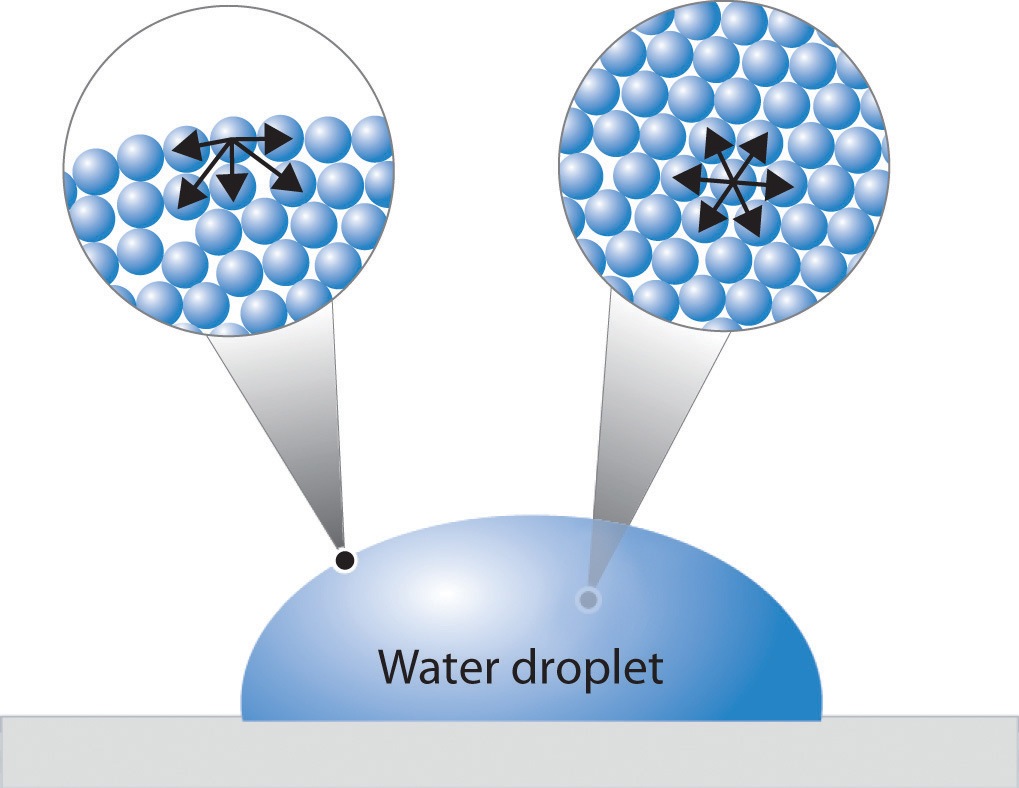
Liquid Surface Tension Formula . The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension results from a sharp change in the density between two adjoined phases or materials. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. A fluid surface has a tendency to occupy as little. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid.
from chem.libretexts.org
Surface tension results from a sharp change in the density between two adjoined phases or materials. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. A fluid surface has a tendency to occupy as little.
Surface Tension Chemistry LibreTexts
Liquid Surface Tension Formula A fluid surface has a tendency to occupy as little. Surface tension results from a sharp change in the density between two adjoined phases or materials. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The molecules on the surface of a liquid are attracted by their neighbors from the. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. A fluid surface has a tendency to occupy as little.
From readchemistry.com
Determination of Surface Tension Read Chemistry Liquid Surface Tension Formula Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is. Liquid Surface Tension Formula.
From www.youtube.com
Excess pressure on a curved surface of liquid // Surface Tension YouTube Liquid Surface Tension Formula Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension results from. Liquid Surface Tension Formula.
From www.learncram.com
Surface Energy Definition, Formula, Units Surface Tension Learn Cram Liquid Surface Tension Formula The molecules on the surface of a liquid are attracted by their neighbors from the. A fluid surface has a tendency to occupy as little. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension results from a sharp change in the density between two adjoined phases or materials.. Liquid Surface Tension Formula.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Liquid Surface Tension Formula The molecules on the surface of a liquid are attracted by their neighbors from the. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The surface tension is force per length and is measured by [n/m]. Liquid Surface Tension Formula.
From www.numerade.com
SOLVED Define surface tension and why we use 2x in the following Liquid Surface Tension Formula Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension results. Liquid Surface Tension Formula.
From byjus.com
Time Period T of a small drop of liquid ( due to surface tension Liquid Surface Tension Formula The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a phenomenon that occurs due. Liquid Surface Tension Formula.
From byjus.com
What is Dimensional Formula of Surface Tension and its Derivation? Liquid Surface Tension Formula The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. A fluid surface has a tendency to occupy as little. Cohesive forces between molecules cause the surface of. Liquid Surface Tension Formula.
From www.youtube.com
Surface Tension on Liquid Jet formula derivation with 3d animation Liquid Surface Tension Formula A fluid surface has a tendency to occupy as little. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension results from a sharp change in the density between two adjoined phases or materials. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The. Liquid Surface Tension Formula.
From www.slideserve.com
PPT FLUID PROPERTIES Chapter 2 PowerPoint Presentation, free download Liquid Surface Tension Formula Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension results from a sharp change in the density between two adjoined phases or materials. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. A fluid surface has a tendency to. Liquid Surface Tension Formula.
From www.chegg.com
Solved Exercise 5. Surface tension y for a liquid in a Liquid Surface Tension Formula A fluid surface has a tendency to occupy as little. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension results from a sharp change in the density between two adjoined phases or materials. The surface tension is force per length and is measured by. Liquid Surface Tension Formula.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Liquid Surface Tension Formula The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. A fluid surface has a tendency to occupy as little. Surface tension is a physical property defined as the amount of force required per unit area. Liquid Surface Tension Formula.
From www.toppr.com
The correct formula to determine surface tension by capillary rise Liquid Surface Tension Formula A fluid surface has a tendency to occupy as little. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is a phenomenon that occurs due to the cohesive forces of. Liquid Surface Tension Formula.
From www.youtube.com
Surface Tension, part 1 Lecture 1.3 Chemical Engineering Fluid Liquid Surface Tension Formula Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension results from a sharp change in the density between two. Liquid Surface Tension Formula.
From www.chegg.com
Solved Q.6 Calculation of Surface Tension from rise in a Liquid Surface Tension Formula Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension results from a sharp change in the density between two adjoined phases or materials. The molecules on the surface of a. Liquid Surface Tension Formula.
From spmphysics.onlinetuition.com.my
Pressure in Liquid SPM Physics Form 4/Form 5 Revision Notes Liquid Surface Tension Formula Surface tension results from a sharp change in the density between two adjoined phases or materials. A fluid surface has a tendency to occupy as little. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. The molecules on the surface of a liquid are attracted by. Liquid Surface Tension Formula.
From www.numerade.com
SOLVED The surface tension of a liquid can be determined by measuring Liquid Surface Tension Formula Surface tension results from a sharp change in the density between two adjoined phases or materials. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. The surface tension. Liquid Surface Tension Formula.
From www.youtube.com
What is Surface Tension Liquid State Liquid Dynamics Surface Liquid Surface Tension Formula The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. A fluid surface has a tendency to occupy as little. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface. Liquid Surface Tension Formula.
From www.youtube.com
Surface Tension, part 2 Lecture 1.4 Chemical Engineering Fluid Liquid Surface Tension Formula A fluid surface has a tendency to occupy as little. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The molecules on the surface of a liquid are attracted by their neighbors. Liquid Surface Tension Formula.
From www.slideserve.com
PPT Lecture 1 Fluid Properties PowerPoint Presentation, free Liquid Surface Tension Formula Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a physical property defined as the amount of force. Liquid Surface Tension Formula.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Liquid Surface Tension Formula Surface tension results from a sharp change in the density between two adjoined phases or materials. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. A fluid surface has a tendency to occupy as little. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface. Liquid Surface Tension Formula.
From www.remet.com
An analysis into Surface Tension The missing link to a good prime Liquid Surface Tension Formula Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension results from a sharp change in the density between two adjoined phases or materials. A fluid surface has a tendency to occupy as little. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The. Liquid Surface Tension Formula.
From www.youtube.com
eUniversityL06M04Surface Tension. Problem 4 Calculate rise of Liquid Surface Tension Formula Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. A fluid surface has a tendency to. Liquid Surface Tension Formula.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free Liquid Surface Tension Formula A fluid surface has a tendency to occupy as little. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension. Liquid Surface Tension Formula.
From civilmint.com
What is Surface Tension Definition of Surface Tension, Examples and Test Liquid Surface Tension Formula Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. A fluid surface has a tendency to occupy as little. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension is a phenomenon that occurs due. Liquid Surface Tension Formula.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid Liquid Surface Tension Formula Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension results from a sharp change in the density between two adjoined phases or materials. The surface tension is force per length and is measured by. Liquid Surface Tension Formula.
From byjus.com
An air bubble inside a liquid surface tension 1*10^ 3 nm grows from a Liquid Surface Tension Formula Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension results from a sharp change in the density between two adjoined phases or materials. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. A fluid surface has a tendency to occupy as little.. Liquid Surface Tension Formula.
From journals.sagepub.com
Archimedes’ principle with surface tension effects in undergraduate Liquid Surface Tension Formula Surface tension results from a sharp change in the density between two adjoined phases or materials. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. A fluid surface has a tendency to occupy as little. Surface tension is a phenomenon that occurs due to the cohesive. Liquid Surface Tension Formula.
From www.sciencetopia.net
Calculate the dimensional formula of surface tension Sciencetopia Liquid Surface Tension Formula Surface tension results from a sharp change in the density between two adjoined phases or materials. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is a physical property defined as the amount. Liquid Surface Tension Formula.
From civilquery.com
What is Surface Tension Definition, Examples and Tests Civil Query Liquid Surface Tension Formula Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. The surface tension is. Liquid Surface Tension Formula.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free Liquid Surface Tension Formula The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. A fluid surface has a tendency to occupy as little. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension results from a sharp change in. Liquid Surface Tension Formula.
From www.face-kyowa.co.jp
What is Surface Tension?:Kyowa Interface Science Liquid Surface Tension Formula Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a. Liquid Surface Tension Formula.
From byjus.com
as we know surface tension = force / length. then what is s= pgrh/2 the Liquid Surface Tension Formula Surface tension results from a sharp change in the density between two adjoined phases or materials. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. A fluid surface has a tendency to occupy as little. Surface tension is a physical property defined as the amount of force required per unit. Liquid Surface Tension Formula.
From www.vrogue.co
Surface Tension Definition Formula Unit Causes Exampl vrogue.co Liquid Surface Tension Formula Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. A fluid surface has a tendency to occupy as little. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible. Liquid Surface Tension Formula.
From pressbooks.online.ucf.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Liquid Surface Tension Formula The surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension results. Liquid Surface Tension Formula.
From www.youtube.com
SURFACE TENSION ON LIQUID JET BASIC OF FLUID MECHANICS 24 ANUNIVERSE Liquid Surface Tension Formula Surface tension results from a sharp change in the density between two adjoined phases or materials. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. A fluid surface has a tendency to occupy as little. Cohesive forces between molecules cause the surface of a liquid to. Liquid Surface Tension Formula.