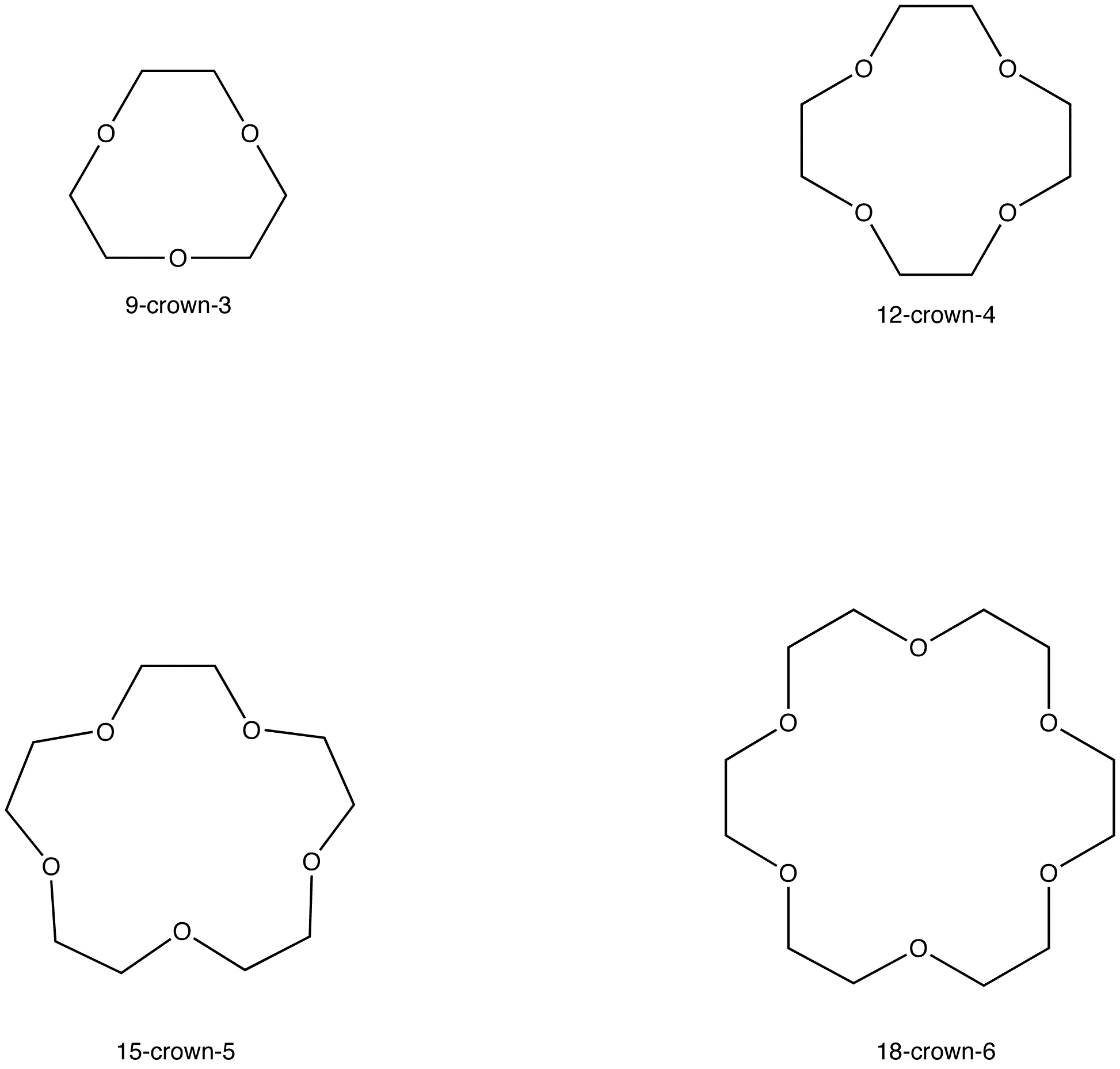
Crown Ethers Form Complexes With Alkali Metals . They form cationic complexes that. Since the external surface of the macrocyclic ligands comprises of organic residue (e.g., ch 2 groups) the complexes are soluble in organic. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. The polyether forms complexes selectively with the alkali metal ions. The size of the ring opening in the crown determines the size of the metal ions which may be accommodated.
from chem.libretexts.org
Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. Since the external surface of the macrocyclic ligands comprises of organic residue (e.g., ch 2 groups) the complexes are soluble in organic. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. The polyether forms complexes selectively with the alkali metal ions. The size of the ring opening in the crown determines the size of the metal ions which may be accommodated. They form cationic complexes that. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of.
Crown Ether Chemistry LibreTexts
Crown Ethers Form Complexes With Alkali Metals Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. They form cationic complexes that. Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. Since the external surface of the macrocyclic ligands comprises of organic residue (e.g., ch 2 groups) the complexes are soluble in organic. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. The polyether forms complexes selectively with the alkali metal ions. The size of the ring opening in the crown determines the size of the metal ions which may be accommodated. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of.
From www.mdpi.com
Catalysts Free FullText Nucleophilic Reactions Using Alkali Metal Fluorides Activated by Crown Ethers Form Complexes With Alkali Metals The polyether forms complexes selectively with the alkali metal ions. Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. Since the external surface of the macrocyclic ligands comprises of organic residue (e.g.,. Crown Ethers Form Complexes With Alkali Metals.
From www.researchgate.net
a,b) Schematic of the plating/stripping behavior for alkali‐metal ions... Download Scientific Crown Ethers Form Complexes With Alkali Metals The polyether forms complexes selectively with the alkali metal ions. Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. They form cationic complexes that. Since the external surface of the macrocyclic ligands comprises of organic residue (e.g., ch 2 groups) the complexes are soluble in organic. Crown ethers prefer to. Crown Ethers Form Complexes With Alkali Metals.
From www.mdpi.com
Catalysts Free FullText Nucleophilic Reactions Using Alkali Metal Fluorides Activated by Crown Ethers Form Complexes With Alkali Metals Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. The polyether forms complexes selectively with the alkali metal ions. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. They form cationic complexes that. Since the. Crown Ethers Form Complexes With Alkali Metals.
From www.semanticscholar.org
Figure 2 from Thermodynamic analysis of alkali metal complex formation of polymerbonded crown Crown Ethers Form Complexes With Alkali Metals Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. Since the external surface of the macrocyclic ligands comprises of organic residue (e.g., ch 2 groups) the complexes are soluble in organic. Crown ethers prefer to bind alkali metal cations with sizes that match that of their. Crown Ethers Form Complexes With Alkali Metals.
From slideplayer.com
Ethers & Epoxides Unit ppt download Crown Ethers Form Complexes With Alkali Metals Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. They form cationic complexes that. The size of the ring opening in the crown determines the size of the metal ions which may be accommodated. Macrocyclic compounds such as crown ethers and cryptands that are selective for. Crown Ethers Form Complexes With Alkali Metals.
From www.researchgate.net
Proposed mechanism for the alkali metal magnesiate + crown ether... Download Scientific Diagram Crown Ethers Form Complexes With Alkali Metals Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does.. Crown Ethers Form Complexes With Alkali Metals.
From www.semanticscholar.org
[PDF] Crown Ethers and Their Alkali Metal Ion Complexes as Assembler Groups in Crown Ethers Form Complexes With Alkali Metals Since the external surface of the macrocyclic ligands comprises of organic residue (e.g., ch 2 groups) the complexes are soluble in organic. They form cationic complexes that. Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown. Crown Ethers Form Complexes With Alkali Metals.
From www.semanticscholar.org
[PDF] Crown Ethers and Their Alkali Metal Ion Complexes as Assembler Groups in Crown Ethers Form Complexes With Alkali Metals Since the external surface of the macrocyclic ligands comprises of organic residue (e.g., ch 2 groups) the complexes are soluble in organic. Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. The size of. Crown Ethers Form Complexes With Alkali Metals.
From www.semanticscholar.org
Figure 3 from Emergence of symmetry and chirality in crown ether complexes with alkali metal Crown Ethers Form Complexes With Alkali Metals Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. They form cationic complexes that. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic. Crown Ethers Form Complexes With Alkali Metals.
From www.semanticscholar.org
Figure 1 from Thermodynamic analysis of alkali metal complex formation of polymerbonded crown Crown Ethers Form Complexes With Alkali Metals Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. The polyether forms. Crown Ethers Form Complexes With Alkali Metals.
From www.researchgate.net
(PDF) Crown Ether Complexes of AlkaliMetal Chlorides from SO2 Crown Ethers Form Complexes With Alkali Metals They form cationic complexes that. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. Crown ethers prefer to bind alkali metal cations with sizes that match that of. Crown Ethers Form Complexes With Alkali Metals.
From chem.libretexts.org
Crown Ether Chemistry LibreTexts Crown Ethers Form Complexes With Alkali Metals Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. The polyether forms complexes selectively with the alkali metal ions. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. Until now the complexation behaviour of alkali and alkaline earth metal ions with. Crown Ethers Form Complexes With Alkali Metals.
From slideplayer.com
Presented by Sheetal Ingulkar For F.Y.B.Sc. ppt download Crown Ethers Form Complexes With Alkali Metals Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. They form cationic complexes that. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. The polyether forms complexes selectively with the alkali metal ions. Macrocyclic compounds such as crown ethers and cryptands. Crown Ethers Form Complexes With Alkali Metals.
From chem.libretexts.org
14.10 Crown Ethers Chemistry LibreTexts Crown Ethers Form Complexes With Alkali Metals The size of the ring opening in the crown determines the size of the metal ions which may be accommodated. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types. Crown Ethers Form Complexes With Alkali Metals.
From www.semanticscholar.org
Figure 10 from Development of the azacrown ether metal complexes as artificial hydrolase Crown Ethers Form Complexes With Alkali Metals The polyether forms complexes selectively with the alkali metal ions. Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic. Crown Ethers Form Complexes With Alkali Metals.
From www.researchgate.net
(PDF) Crown Ether Complexes of AlkaliMetal Chlorides from SO2 Crown Ethers Form Complexes With Alkali Metals They form cationic complexes that. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. The size of the ring opening in the crown determines the size of the metal ions which. Crown Ethers Form Complexes With Alkali Metals.
From pubs.acs.org
Stable AlkaliMetal Complexes of Hybrid DisilaCrown Ethers Chemistry Crown Ethers Form Complexes With Alkali Metals They form cationic complexes that. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. The polyether forms complexes selectively with the alkali metal ions. Since the external surface of the macrocyclic ligands comprises of organic residue (e.g., ch 2 groups) the complexes are soluble in organic. Until now the complexation behaviour. Crown Ethers Form Complexes With Alkali Metals.
From www.semanticscholar.org
Figure 1 from Evaluation of the Hydrophilic Properties of Crown Etherionpair Complexes with Crown Ethers Form Complexes With Alkali Metals They form cationic complexes that. Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has. Crown Ethers Form Complexes With Alkali Metals.
From www.researchgate.net
a) Crystal structures of different crown ethers and their complexes... Download Scientific Diagram Crown Ethers Form Complexes With Alkali Metals The polyether forms complexes selectively with the alkali metal ions. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. The size of the ring opening in. Crown Ethers Form Complexes With Alkali Metals.
From www.semanticscholar.org
Figure 2 from Transfer activity coefficients of crown ethers and their alkali metal ion Crown Ethers Form Complexes With Alkali Metals The polyether forms complexes selectively with the alkali metal ions. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. They form cationic complexes that. Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. The size of the ring opening. Crown Ethers Form Complexes With Alkali Metals.
From www.mdpi.com
Catalysts Free FullText Nucleophilic Reactions Using Alkali Metal Fluorides Activated by Crown Ethers Form Complexes With Alkali Metals Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. The size of the ring opening in the crown determines the size of the metal ions which may be accommodated. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds,. Crown Ethers Form Complexes With Alkali Metals.
From www.researchgate.net
Some of the crown ethers that form complexes with C 60 . Download Scientific Diagram Crown Ethers Form Complexes With Alkali Metals Since the external surface of the macrocyclic ligands comprises of organic residue (e.g., ch 2 groups) the complexes are soluble in organic. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. Until. Crown Ethers Form Complexes With Alkali Metals.
From www.researchgate.net
Optimized structures of the crown4 ethers complexing with Li + and Na... Download Scientific Crown Ethers Form Complexes With Alkali Metals Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. The polyether forms complexes selectively with the alkali metal ions. Since the external surface of the macrocyclic ligands comprises of organic residue (e.g., ch 2 groups) the complexes are soluble in organic. They form cationic complexes that. Crown ethers prefer to bind. Crown Ethers Form Complexes With Alkali Metals.
From www.semanticscholar.org
Figure 1 from Transfer activity coefficients of crown ethers and their alkali metal ion Crown Ethers Form Complexes With Alkali Metals Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. They form cationic complexes that. Since. Crown Ethers Form Complexes With Alkali Metals.
From www.researchgate.net
(PDF) Amidewater hydrogenbond motifs in alkalimetal/crown ether complexes of Crown Ethers Form Complexes With Alkali Metals Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. Ammonium. Crown Ethers Form Complexes With Alkali Metals.
From slidetodoc.com
Organic Chemistry 9 th Edition L G Wade Crown Ethers Form Complexes With Alkali Metals The polyether forms complexes selectively with the alkali metal ions. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. Macrocyclic compounds such as crown ethers and. Crown Ethers Form Complexes With Alkali Metals.
From slideplayer.com
Dnyanasadhana College, Thane. Department of Chemistry M. Sc ppt download Crown Ethers Form Complexes With Alkali Metals The polyether forms complexes selectively with the alkali metal ions. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. The size of the ring opening in the crown determines the size of the metal ions which may be accommodated. Since the external surface of the macrocyclic ligands comprises of organic residue. Crown Ethers Form Complexes With Alkali Metals.
From favpng.com
Crown Ether 18Crown6 Alkali Metal Potassium, PNG, 602x600px, Ether, Alkali Metal, Cation Crown Ethers Form Complexes With Alkali Metals Since the external surface of the macrocyclic ligands comprises of organic residue (e.g., ch 2 groups) the complexes are soluble in organic. They form cationic complexes that. The polyether forms complexes selectively with the alkali metal ions. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. The size of. Crown Ethers Form Complexes With Alkali Metals.
From www.semanticscholar.org
Table 1 from Evaluation of the Hydrophilic Properties of Crown Etherionpair Complexes with Crown Ethers Form Complexes With Alkali Metals Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. The polyether forms complexes selectively with the alkali metal ions. They form cationic complexes that. Since the external surface of. Crown Ethers Form Complexes With Alkali Metals.
From www.mdpi.com
Applied Sciences Free FullText Heterocyclic Crown Ethers with Potential Biological and Crown Ethers Form Complexes With Alkali Metals Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of.. Crown Ethers Form Complexes With Alkali Metals.
From www.researchgate.net
Scheme 2 Synthesis of new doublearmed crown ethers (15) Download Scientific Diagram Crown Ethers Form Complexes With Alkali Metals Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. They form cationic complexes that. Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal. Crown Ethers Form Complexes With Alkali Metals.
From www.youtube.com
polyether complexes of alkali metals (crown ethers and crypt) YouTube Crown Ethers Form Complexes With Alkali Metals Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. The size of the ring opening in the crown determines the size of the metal ions which may be accommodated. Since the external surface. Crown Ethers Form Complexes With Alkali Metals.
From www.youtube.com
Crown Ethers YouTube Crown Ethers Form Complexes With Alkali Metals The polyether forms complexes selectively with the alkali metal ions. They form cationic complexes that. Macrocyclic compounds such as crown ethers and cryptands that are selective for particular alkali metal ions have been synthesized. Ammonium shares with alkali metal cations the tendency to form stable complexes with crown ethers and cryptands, but it does. Since the external surface of the. Crown Ethers Form Complexes With Alkali Metals.
From www.researchgate.net
The cationic parts of selected complexes with crown ether... Download Scientific Diagram Crown Ethers Form Complexes With Alkali Metals Since the external surface of the macrocyclic ligands comprises of organic residue (e.g., ch 2 groups) the complexes are soluble in organic. Until now the complexation behaviour of alkali and alkaline earth metal ions with crown ethers has been extensively studied in various types of. Crown ethers prefer to bind alkali metal cations with sizes that match that of their. Crown Ethers Form Complexes With Alkali Metals.
From www.youtube.com
Alkali Metals Important Concepts, Complexation with Crown Ethers and Cryptands YouTube Crown Ethers Form Complexes With Alkali Metals Electrostatic interactions also allow alkali metal ions to form complexes with certain cyclic polyethers and related compounds, such. The polyether forms complexes selectively with the alkali metal ions. The size of the ring opening in the crown determines the size of the metal ions which may be accommodated. Until now the complexation behaviour of alkali and alkaline earth metal ions. Crown Ethers Form Complexes With Alkali Metals.