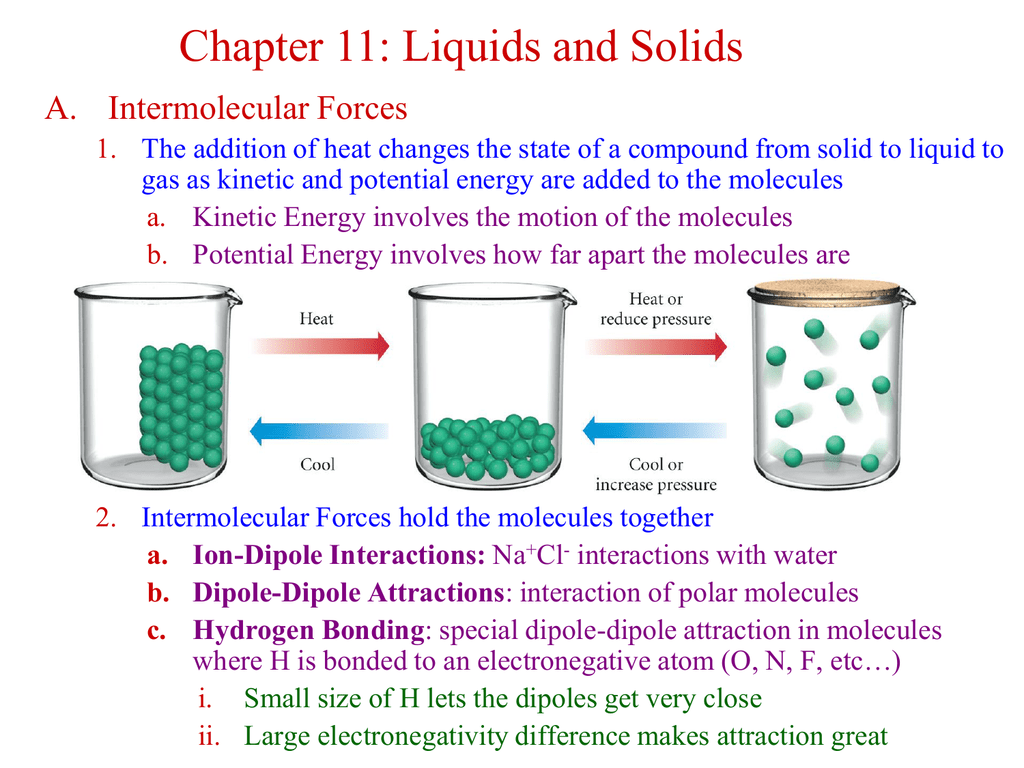
Solid To Liquid Potential Energy . watch different types of molecules form a solid, liquid, or gas. changes in a material's temperature or state of matter are caused by changes to the internal energy. for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). the conversion of a solid to a liquid is called fusion (or melting). And removing energy from liquid water causes. Add or remove heat and watch the phase change. endothermic processes go from low energy to high energy: liquids have more kinetic energy than solids. transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the. Solid to liquid to gas. the energy released by chemical reaction is not directly related to the state (solid or liquid) but to the chemistry of the material. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. The energy required to melt 1 mol of a substance is its enthalpy. Some of these particles will have enough kinetic energy to break their liquid bonds and escape as a gas (evaporation). Exothermic processes go from high energy to low energy:
from studylib.net
watch different types of molecules form a solid, liquid, or gas. The energy required to melt 1 mol of a substance is its enthalpy. Some of these particles will have enough kinetic energy to break their liquid bonds and escape as a gas (evaporation). the energy released by chemical reaction is not directly related to the state (solid or liquid) but to the chemistry of the material. Change the temperature or volume of. Add or remove heat and watch the phase change. changes in a material's temperature or state of matter are caused by changes to the internal energy. Solid to liquid to gas. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. the conversion of a solid to a liquid is called fusion (or melting).
Chapter 11 Liquids and Solids A. Intermolecular Forces
Solid To Liquid Potential Energy watch different types of molecules form a solid, liquid, or gas. Some of these particles will have enough kinetic energy to break their liquid bonds and escape as a gas (evaporation). The energy required to melt 1 mol of a substance is its enthalpy. watch different types of molecules form a solid, liquid, or gas. Change the temperature or volume of. And removing energy from liquid water causes. Add or remove heat and watch the phase change. the conversion of a solid to a liquid is called fusion (or melting). Solid to liquid to gas. transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the. changes in a material's temperature or state of matter are caused by changes to the internal energy. liquids have more kinetic energy than solids. the energy released by chemical reaction is not directly related to the state (solid or liquid) but to the chemistry of the material. endothermic processes go from low energy to high energy: Exothermic processes go from high energy to low energy: for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas).
From www.pinterest.com
Difference between Solid, Liquid and Gas in Tabular form. States of Solid To Liquid Potential Energy watch different types of molecules form a solid, liquid, or gas. The energy required to melt 1 mol of a substance is its enthalpy. Change the temperature or volume of. changes in a material's temperature or state of matter are caused by changes to the internal energy. endothermic processes go from low energy to high energy: If. Solid To Liquid Potential Energy.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Solid To Liquid Potential Energy endothermic processes go from low energy to high energy: Exothermic processes go from high energy to low energy: the energy released by chemical reaction is not directly related to the state (solid or liquid) but to the chemistry of the material. Solid to liquid to gas. Add or remove heat and watch the phase change. And removing energy. Solid To Liquid Potential Energy.
From sciencetallis.weebly.com
3. Particle Model of Matter THOMAS TALLIS SCIENCE Solid To Liquid Potential Energy the energy released by chemical reaction is not directly related to the state (solid or liquid) but to the chemistry of the material. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. Change the temperature or volume of. And removing energy from liquid water causes. transitions. Solid To Liquid Potential Energy.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid To Liquid Potential Energy The energy required to melt 1 mol of a substance is its enthalpy. changes in a material's temperature or state of matter are caused by changes to the internal energy. transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the. And removing energy from liquid water causes. liquids have more kinetic. Solid To Liquid Potential Energy.
From www.docbrown.info
GASES LIQUIDS SOLIDS States of Matter, particle theory models Solid To Liquid Potential Energy for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). changes in a material's temperature or state of matter are caused by changes to the internal energy. Exothermic processes go from high energy to low energy: The energy required to melt 1 mol of a substance is its enthalpy. Add. Solid To Liquid Potential Energy.
From chemistry.stackexchange.com
electrochemistry Why does aqueous NaCl conduct electricity Solid To Liquid Potential Energy Exothermic processes go from high energy to low energy: changes in a material's temperature or state of matter are caused by changes to the internal energy. for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). Add or remove heat and watch the phase change. the energy released by. Solid To Liquid Potential Energy.
From www.gasrebate.net
Properties Of Solids Liquids Gases Compared Teachoo Science Solid To Liquid Potential Energy Add or remove heat and watch the phase change. changes in a material's temperature or state of matter are caused by changes to the internal energy. The energy required to melt 1 mol of a substance is its enthalpy. watch different types of molecules form a solid, liquid, or gas. Exothermic processes go from high energy to low. Solid To Liquid Potential Energy.
From www.slideserve.com
PPT Chemical Potential for Liquid/Solid Solutions PowerPoint Solid To Liquid Potential Energy endothermic processes go from low energy to high energy: changes in a material's temperature or state of matter are caused by changes to the internal energy. Add or remove heat and watch the phase change. Solid to liquid to gas. for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a. Solid To Liquid Potential Energy.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at Solid To Liquid Potential Energy The energy required to melt 1 mol of a substance is its enthalpy. And removing energy from liquid water causes. Add or remove heat and watch the phase change. the energy released by chemical reaction is not directly related to the state (solid or liquid) but to the chemistry of the material. watch different types of molecules form. Solid To Liquid Potential Energy.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Solid To Liquid Potential Energy the conversion of a solid to a liquid is called fusion (or melting). transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the. for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). And removing energy from liquid water causes. watch different. Solid To Liquid Potential Energy.
From igcsechemistryrevision.weebly.com
iGCSE CHEMISTRY REVISION HELP Particles & Equations Solid To Liquid Potential Energy And removing energy from liquid water causes. changes in a material's temperature or state of matter are caused by changes to the internal energy. Exothermic processes go from high energy to low energy: endothermic processes go from low energy to high energy: If you add heat energy to a liquid, the particles will move faster around each other. Solid To Liquid Potential Energy.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Solid To Liquid Potential Energy The energy required to melt 1 mol of a substance is its enthalpy. Some of these particles will have enough kinetic energy to break their liquid bonds and escape as a gas (evaporation). watch different types of molecules form a solid, liquid, or gas. liquids have more kinetic energy than solids. Add or remove heat and watch the. Solid To Liquid Potential Energy.
From www.youtube.com
Gibbs Free Energy of Mixing and LiquidLiquid Separation YouTube Solid To Liquid Potential Energy The energy required to melt 1 mol of a substance is its enthalpy. And removing energy from liquid water causes. the energy released by chemical reaction is not directly related to the state (solid or liquid) but to the chemistry of the material. transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to. Solid To Liquid Potential Energy.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Solid To Liquid Potential Energy watch different types of molecules form a solid, liquid, or gas. transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the. for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). Exothermic processes go from high energy to low energy: endothermic processes. Solid To Liquid Potential Energy.
From www.shutterstock.com
2,258 Motion Of Particles Of Matter Images, Stock Photos & Vectors Solid To Liquid Potential Energy changes in a material's temperature or state of matter are caused by changes to the internal energy. Exothermic processes go from high energy to low energy: Some of these particles will have enough kinetic energy to break their liquid bonds and escape as a gas (evaporation). The energy required to melt 1 mol of a substance is its enthalpy.. Solid To Liquid Potential Energy.
From saylordotorg.github.io
Liquids Solid To Liquid Potential Energy watch different types of molecules form a solid, liquid, or gas. Add or remove heat and watch the phase change. for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). The energy required to melt 1 mol of a substance is its enthalpy. Some of these particles will have enough. Solid To Liquid Potential Energy.
From www.teachoo.com
Difference between solids and liquids conducting electricity Teachoo Solid To Liquid Potential Energy watch different types of molecules form a solid, liquid, or gas. the conversion of a solid to a liquid is called fusion (or melting). for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). endothermic processes go from low energy to high energy: Some of these particles will. Solid To Liquid Potential Energy.
From studylib.net
STATES OF MATTER VENN DIAGRAM Solid To Liquid Potential Energy And removing energy from liquid water causes. Exothermic processes go from high energy to low energy: transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the. Solid to liquid to gas. for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). endothermic processes. Solid To Liquid Potential Energy.
From present5.com
Gases Liquids and Solids The Phases or States Solid To Liquid Potential Energy endothermic processes go from low energy to high energy: the conversion of a solid to a liquid is called fusion (or melting). for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). And removing energy from liquid water causes. If you add heat energy to a liquid, the particles. Solid To Liquid Potential Energy.
From present5.com
Gases Liquids and Solids The Phases or States Solid To Liquid Potential Energy transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the. Exothermic processes go from high energy to low energy: watch different types of molecules form a solid, liquid, or gas. changes in a material's temperature or state of matter are caused by changes to the internal energy. Change the temperature or. Solid To Liquid Potential Energy.
From www.elevise.co.uk
C2 J) Changing State AQA Combined Science Trilogy Elevise Solid To Liquid Potential Energy transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the. Add or remove heat and watch the phase change. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. Solid to liquid to gas. the conversion of a solid to a. Solid To Liquid Potential Energy.
From gcsephysicsninja.com
3. Energy of solids, liquids and gases Solid To Liquid Potential Energy watch different types of molecules form a solid, liquid, or gas. Change the temperature or volume of. Some of these particles will have enough kinetic energy to break their liquid bonds and escape as a gas (evaporation). Exothermic processes go from high energy to low energy: Add or remove heat and watch the phase change. And removing energy from. Solid To Liquid Potential Energy.
From stock.adobe.com
Thermal expansion of solids and liquids. The tendency of materials to Solid To Liquid Potential Energy Solid to liquid to gas. Add or remove heat and watch the phase change. Exothermic processes go from high energy to low energy: for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). Some of these particles will have enough kinetic energy to break their liquid bonds and escape as a. Solid To Liquid Potential Energy.
From shaunmwilliams.com
Lecture 1 Presentation Solid To Liquid Potential Energy Exothermic processes go from high energy to low energy: Some of these particles will have enough kinetic energy to break their liquid bonds and escape as a gas (evaporation). the conversion of a solid to a liquid is called fusion (or melting). And removing energy from liquid water causes. watch different types of molecules form a solid, liquid,. Solid To Liquid Potential Energy.
From dxocetkkj.blob.core.windows.net
Heating And Cooling Curves Phase Diagrams at Beatrice Smart blog Solid To Liquid Potential Energy the conversion of a solid to a liquid is called fusion (or melting). changes in a material's temperature or state of matter are caused by changes to the internal energy. the energy released by chemical reaction is not directly related to the state (solid or liquid) but to the chemistry of the material. The energy required to. Solid To Liquid Potential Energy.
From wulixb.iphy.ac.cn
非对称纳米通道内界面热阻的分子动力学研究 Solid To Liquid Potential Energy Add or remove heat and watch the phase change. the conversion of a solid to a liquid is called fusion (or melting). Solid to liquid to gas. liquids have more kinetic energy than solids. for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). Exothermic processes go from high. Solid To Liquid Potential Energy.
From www.researchgate.net
diagrams for (a) ideal solution, (b) and (c Solid To Liquid Potential Energy endothermic processes go from low energy to high energy: And removing energy from liquid water causes. Some of these particles will have enough kinetic energy to break their liquid bonds and escape as a gas (evaporation). watch different types of molecules form a solid, liquid, or gas. for example, adding thermal energy (heat) to liquid water causes. Solid To Liquid Potential Energy.
From mavink.com
Changing States Of Matter Diagram Solid To Liquid Potential Energy The energy required to melt 1 mol of a substance is its enthalpy. the energy released by chemical reaction is not directly related to the state (solid or liquid) but to the chemistry of the material. Change the temperature or volume of. If you add heat energy to a liquid, the particles will move faster around each other as. Solid To Liquid Potential Energy.
From school.freekaoyan.com
非对称纳米通道内界面热阻的分子动力学研究 中科院物理研究所 Free考研考试 Solid To Liquid Potential Energy liquids have more kinetic energy than solids. Change the temperature or volume of. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. And removing energy from liquid water causes. The energy required to melt 1 mol of a substance is its enthalpy. Exothermic processes go from high. Solid To Liquid Potential Energy.
From www.snexplores.org
Explainer What are the different states of matter? Solid To Liquid Potential Energy liquids have more kinetic energy than solids. Exothermic processes go from high energy to low energy: If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. Add or remove heat and watch the phase change. the energy released by chemical reaction is not directly related to the. Solid To Liquid Potential Energy.
From byjus.com
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law Solid To Liquid Potential Energy If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. And removing energy from liquid water causes. watch different types of molecules form a solid, liquid, or gas. liquids have more kinetic energy than solids. transitions between solid, liquid, and gaseous phases typically involve large amounts. Solid To Liquid Potential Energy.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid To Liquid Potential Energy changes in a material's temperature or state of matter are caused by changes to the internal energy. the conversion of a solid to a liquid is called fusion (or melting). watch different types of molecules form a solid, liquid, or gas. Some of these particles will have enough kinetic energy to break their liquid bonds and escape. Solid To Liquid Potential Energy.
From studymind.co.uk
Changing State (GCSE Chemistry) Study Mind Solid To Liquid Potential Energy Exothermic processes go from high energy to low energy: And removing energy from liquid water causes. Add or remove heat and watch the phase change. endothermic processes go from low energy to high energy: changes in a material's temperature or state of matter are caused by changes to the internal energy. The energy required to melt 1 mol. Solid To Liquid Potential Energy.
From www.vecteezy.com
States of matter, Solid, liquid, gas varying particle arrangement and Solid To Liquid Potential Energy changes in a material's temperature or state of matter are caused by changes to the internal energy. Some of these particles will have enough kinetic energy to break their liquid bonds and escape as a gas (evaporation). the conversion of a solid to a liquid is called fusion (or melting). Change the temperature or volume of. for. Solid To Liquid Potential Energy.
From www.slideserve.com
PPT States of Matter A. The Theory PowerPoint Presentation Solid To Liquid Potential Energy watch different types of molecules form a solid, liquid, or gas. for example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). The energy required to melt 1 mol of a substance is its enthalpy. liquids have more kinetic energy than solids. Some of these particles will have enough kinetic. Solid To Liquid Potential Energy.