Laboratory Preparation Of Iron (Iii) Chloride . Anhydrous iron (iii) chloride may be prepared by union of the elements: (i) substance b is a dehydrating agent. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). (ii) anhydrous calciu m chloride absor bs the moisture present in the. This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and using colorimetric analysis to determine unknown. (ii) b prevents the entry of moisture inside the apparatus because it. To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 % purity) in deionized or. Ferric chloride test is conducted to determine the. (i) substance b is anhydrous (fused) calcium chloride (cacl 2).
from www.numerade.com
(ii) anhydrous calciu m chloride absor bs the moisture present in the. To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 % purity) in deionized or. Ferric chloride test is conducted to determine the. [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and using colorimetric analysis to determine unknown. (i) substance b is a dehydrating agent. Anhydrous iron (iii) chloride may be prepared by union of the elements: (i) substance b is anhydrous (fused) calcium chloride (cacl 2). (ii) b prevents the entry of moisture inside the apparatus because it.
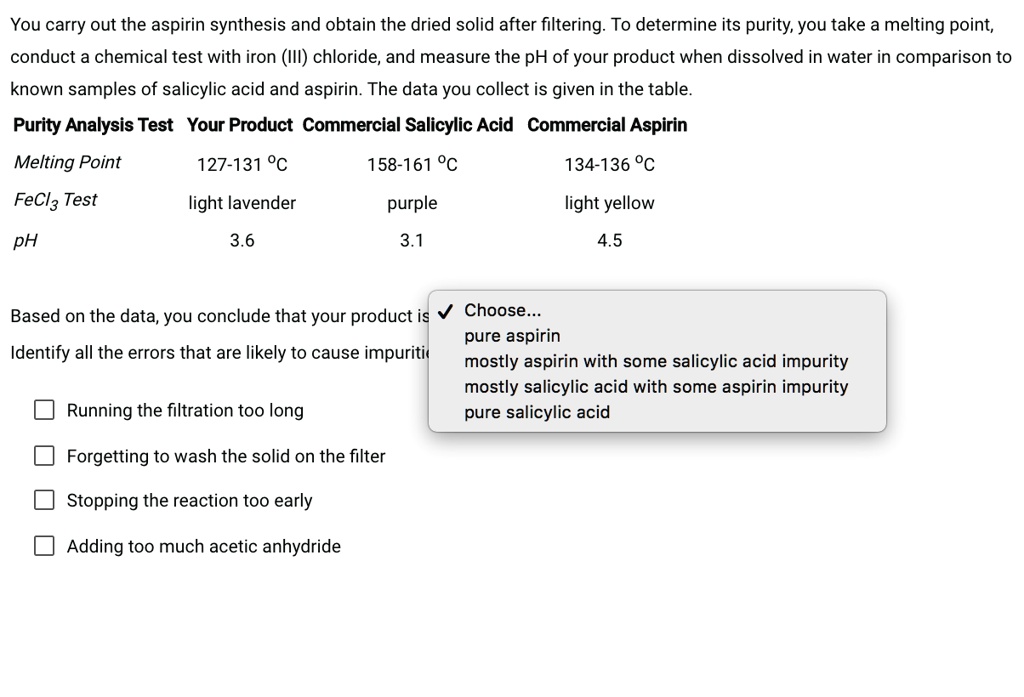
SOLVED You carry out the aspirin synthesis and obtain the dried solid
Laboratory Preparation Of Iron (Iii) Chloride This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and using colorimetric analysis to determine unknown. To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 % purity) in deionized or. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. Anhydrous iron (iii) chloride may be prepared by union of the elements: This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and using colorimetric analysis to determine unknown. (i) substance b is a dehydrating agent. (ii) anhydrous calciu m chloride absor bs the moisture present in the. Ferric chloride test is conducted to determine the. [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). (ii) b prevents the entry of moisture inside the apparatus because it. (i) substance b is anhydrous (fused) calcium chloride (cacl 2).
From www.youtube.com
Aspirin Purity Test Ferric Chloride YouTube Laboratory Preparation Of Iron (Iii) Chloride To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 % purity) in deionized or. This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and using colorimetric analysis to determine unknown. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine.. Laboratory Preparation Of Iron (Iii) Chloride.
From www.chegg.com
Solved Synthesis of Aspirin experiment 2. Test for purity of Laboratory Preparation Of Iron (Iii) Chloride [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). (i) substance b is a dehydrating agent. (i) substance b is anhydrous (fused) calcium chloride (cacl 2). Ferric chloride test is conducted to determine the. Anhydrous iron (iii) chloride may be prepared by union of the elements: This experiment involves preparing standard solutions of iron (iii). Laboratory Preparation Of Iron (Iii) Chloride.
From www.youtube.com
FeCl3 Iron 3 Chloride FeCl3 anhydrous FeCl3 Solution Laboratory Preparation Of Iron (Iii) Chloride (i) substance b is anhydrous (fused) calcium chloride (cacl 2). To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 % purity) in deionized or. (ii) anhydrous calciu m chloride absor bs the moisture present in the. [2] 2 fe (s) + 3 cl 2 (g) → 2. Laboratory Preparation Of Iron (Iii) Chloride.
From www.chemedx.org
Demonstrating the Colors of Transition Metal Complex Ions Chemical Laboratory Preparation Of Iron (Iii) Chloride This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and using colorimetric analysis to determine unknown. Anhydrous iron (iii) chloride may be prepared by union of the elements: (ii) anhydrous calciu m chloride absor bs the moisture present in the. (i) substance b is anhydrous (fused) calcium chloride (cacl 2). To prepare 1000 ml of a. Laboratory Preparation Of Iron (Iii) Chloride.
From www.xunjiesemimaterials.com
Iron(Ii) Chloride(FeCl2) Laboratory Preparation Of Iron (Iii) Chloride (ii) b prevents the entry of moisture inside the apparatus because it. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. (i) substance b is anhydrous (fused) calcium chloride (cacl 2). (i) substance b is a dehydrating agent. (ii) anhydrous calciu m chloride absor bs the moisture present in the. This experiment involves preparing standard solutions. Laboratory Preparation Of Iron (Iii) Chloride.
From www.researchgate.net
What is the chemical difference between these iron III chloride solutions? Laboratory Preparation Of Iron (Iii) Chloride To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 % purity) in deionized or. (ii) anhydrous calciu m chloride absor bs the moisture present in the. [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). Anhydrous iron (iii) chloride may be prepared. Laboratory Preparation Of Iron (Iii) Chloride.
From www.researchgate.net
(A) Schematic presentation of synthesis of Iron oxide nanoparticles by Laboratory Preparation Of Iron (Iii) Chloride (ii) b prevents the entry of moisture inside the apparatus because it. (i) substance b is anhydrous (fused) calcium chloride (cacl 2). Ferric chloride test is conducted to determine the. To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 % purity) in deionized or. Anhydrous iron (iii). Laboratory Preparation Of Iron (Iii) Chloride.
From 2022.markettraders.com
Ferric Chloride Formula, Solution Preparation And Ferric, 49 OFF Laboratory Preparation Of Iron (Iii) Chloride (ii) b prevents the entry of moisture inside the apparatus because it. Ferric chloride test is conducted to determine the. [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. (i) substance b is anhydrous (fused) calcium chloride (cacl 2). (ii) anhydrous calciu. Laboratory Preparation Of Iron (Iii) Chloride.
From eureka.patsnap.com
Method of preparing iron oxide nanoparticles Eureka Patsnap develop Laboratory Preparation Of Iron (Iii) Chloride To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 % purity) in deionized or. (i) substance b is anhydrous (fused) calcium chloride (cacl 2). (ii) anhydrous calciu m chloride absor bs the moisture present in the. [2] 2 fe (s) + 3 cl 2 (g) → 2. Laboratory Preparation Of Iron (Iii) Chloride.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Laboratory Preparation Of Iron (Iii) Chloride (ii) b prevents the entry of moisture inside the apparatus because it. (ii) anhydrous calciu m chloride absor bs the moisture present in the. Anhydrous iron (iii) chloride may be prepared by union of the elements: (i) substance b is anhydrous (fused) calcium chloride (cacl 2). [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s).. Laboratory Preparation Of Iron (Iii) Chloride.
From www.youtube.com
Ferric Chloride Test for Phenols YouTube Laboratory Preparation Of Iron (Iii) Chloride (i) substance b is anhydrous (fused) calcium chloride (cacl 2). (ii) b prevents the entry of moisture inside the apparatus because it. Ferric chloride test is conducted to determine the. This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and using colorimetric analysis to determine unknown. Anhydrous iron (iii) chloride can be prepared by reacting metallic. Laboratory Preparation Of Iron (Iii) Chloride.
From www.pinterest.com
Chemistry Pals Laboratory Preparation of Hydrogen by the Action of Laboratory Preparation Of Iron (Iii) Chloride Anhydrous iron (iii) chloride may be prepared by union of the elements: (i) substance b is a dehydrating agent. Ferric chloride test is conducted to determine the. [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). (i) substance b is anhydrous (fused) calcium chloride (cacl 2). This experiment involves preparing standard solutions of iron (iii). Laboratory Preparation Of Iron (Iii) Chloride.
From www.chegg.com
Method Synthesis A (cold) iron(III) chloride solution Laboratory Preparation Of Iron (Iii) Chloride [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). Ferric chloride test is conducted to determine the. To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 % purity) in deionized or. (i) substance b is a dehydrating agent. (ii) b prevents the. Laboratory Preparation Of Iron (Iii) Chloride.
From icsechemistry16.blogspot.com
Preparation of Iron (III) chloride (Ferric chloride) Laboratory Preparation Of Iron (Iii) Chloride Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and using colorimetric analysis to determine unknown. (ii) b prevents the entry of moisture inside the apparatus because it. To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have. Laboratory Preparation Of Iron (Iii) Chloride.
From www.researchgate.net
What is the chemical difference between these iron III chloride Laboratory Preparation Of Iron (Iii) Chloride Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. Anhydrous iron (iii) chloride may be prepared by union of the elements: (i) substance b is anhydrous (fused) calcium chloride (cacl 2). To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 % purity) in. Laboratory Preparation Of Iron (Iii) Chloride.
From www.researchgate.net
Preparation of iron oxide nanoparticles. Download Scientific Diagram Laboratory Preparation Of Iron (Iii) Chloride (ii) anhydrous calciu m chloride absor bs the moisture present in the. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). (i) substance b is anhydrous (fused) calcium chloride (cacl 2). This experiment involves preparing standard solutions of iron (iii) chloride at. Laboratory Preparation Of Iron (Iii) Chloride.
From www.studocu.com
Exp 2 lab procedure CHM361 EXPERIMENT 2 Synthesis of Potassium Tris Laboratory Preparation Of Iron (Iii) Chloride Ferric chloride test is conducted to determine the. (ii) anhydrous calciu m chloride absor bs the moisture present in the. (i) substance b is anhydrous (fused) calcium chloride (cacl 2). Anhydrous iron (iii) chloride may be prepared by union of the elements: Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. This experiment involves preparing standard. Laboratory Preparation Of Iron (Iii) Chloride.
From www.researchgate.net
(PDF) Effect of silicon on the synthesis of iron aluminides from Laboratory Preparation Of Iron (Iii) Chloride Ferric chloride test is conducted to determine the. (ii) anhydrous calciu m chloride absor bs the moisture present in the. To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 % purity) in deionized or. (i) substance b is a dehydrating agent. Anhydrous iron (iii) chloride can be. Laboratory Preparation Of Iron (Iii) Chloride.
From www.mdpi.com
Metals Free FullText A Straightforward Route to Tetrachloroauric Laboratory Preparation Of Iron (Iii) Chloride (ii) b prevents the entry of moisture inside the apparatus because it. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. (ii) anhydrous calciu m chloride absor bs the moisture present in the. To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 %. Laboratory Preparation Of Iron (Iii) Chloride.
From www.dreamstime.com
Diagram of Preparation of Hydrogen Chloride Gas Stock Illustration Laboratory Preparation Of Iron (Iii) Chloride (ii) b prevents the entry of moisture inside the apparatus because it. (ii) anhydrous calciu m chloride absor bs the moisture present in the. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. (i) substance b is anhydrous (fused) calcium chloride (cacl 2). This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations. Laboratory Preparation Of Iron (Iii) Chloride.
From www.alamy.com
Labelled diagram for laboratory preparation of hydrogen from zinc and Laboratory Preparation Of Iron (Iii) Chloride To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 % purity) in deionized or. Anhydrous iron (iii) chloride may be prepared by union of the elements: [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). Anhydrous iron (iii) chloride can be prepared. Laboratory Preparation Of Iron (Iii) Chloride.
From www.chegg.com
Method Synthesis A (cold) iron(III) chloride solution Laboratory Preparation Of Iron (Iii) Chloride [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). (i) substance b is a dehydrating agent. This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and using colorimetric analysis to determine unknown. Anhydrous iron (iii) chloride may be prepared by union of the elements: (ii) b prevents the entry of moisture. Laboratory Preparation Of Iron (Iii) Chloride.
From testbook.com
Iron (III) Chloride Formula Know Structure, Preparation,& Uses Laboratory Preparation Of Iron (Iii) Chloride Ferric chloride test is conducted to determine the. (ii) b prevents the entry of moisture inside the apparatus because it. [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). (i) substance b is a dehydrating agent. (ii) anhydrous calciu m chloride absor bs the moisture present in the. (i) substance b is anhydrous (fused) calcium. Laboratory Preparation Of Iron (Iii) Chloride.
From www.youtube.com
Iron(iii) Chloride Preparation YouTube Laboratory Preparation Of Iron (Iii) Chloride (i) substance b is anhydrous (fused) calcium chloride (cacl 2). (ii) anhydrous calciu m chloride absor bs the moisture present in the. This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and using colorimetric analysis to determine unknown. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. Ferric chloride test is conducted. Laboratory Preparation Of Iron (Iii) Chloride.
From www.youtube.com
Iron(III) chloride YouTube Laboratory Preparation Of Iron (Iii) Chloride (ii) b prevents the entry of moisture inside the apparatus because it. Anhydrous iron (iii) chloride may be prepared by union of the elements: (ii) anhydrous calciu m chloride absor bs the moisture present in the. (i) substance b is a dehydrating agent. (i) substance b is anhydrous (fused) calcium chloride (cacl 2). This experiment involves preparing standard solutions of. Laboratory Preparation Of Iron (Iii) Chloride.
From www.coursehero.com
[Solved] Synthesis of iron (III) acetylacetonate complex In a small Laboratory Preparation Of Iron (Iii) Chloride (i) substance b is a dehydrating agent. (ii) b prevents the entry of moisture inside the apparatus because it. Ferric chloride test is conducted to determine the. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and using colorimetric analysis to determine unknown.. Laboratory Preparation Of Iron (Iii) Chloride.
From www.numerade.com
SOLVED You carry out the aspirin synthesis and obtain the dried solid Laboratory Preparation Of Iron (Iii) Chloride (ii) anhydrous calciu m chloride absor bs the moisture present in the. This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and using colorimetric analysis to determine unknown. (i) substance b is anhydrous (fused) calcium chloride (cacl 2). Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. Anhydrous iron (iii) chloride may. Laboratory Preparation Of Iron (Iii) Chloride.
From www.alamy.com
Labelled diagram Stock Vector Images Alamy Laboratory Preparation Of Iron (Iii) Chloride (ii) b prevents the entry of moisture inside the apparatus because it. Anhydrous iron (iii) chloride may be prepared by union of the elements: (ii) anhydrous calciu m chloride absor bs the moisture present in the. (i) substance b is anhydrous (fused) calcium chloride (cacl 2). Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. [2]. Laboratory Preparation Of Iron (Iii) Chloride.
From www.doubtnut.com
The diagram shows an apparatus for the laboratory preparation of h Laboratory Preparation Of Iron (Iii) Chloride (ii) anhydrous calciu m chloride absor bs the moisture present in the. (ii) b prevents the entry of moisture inside the apparatus because it. (i) substance b is a dehydrating agent. Ferric chloride test is conducted to determine the. To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o. Laboratory Preparation Of Iron (Iii) Chloride.
From www.youtube.com
Laboratory Preparation of Chlorine YouTube Laboratory Preparation Of Iron (Iii) Chloride (i) substance b is anhydrous (fused) calcium chloride (cacl 2). Ferric chloride test is conducted to determine the. [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). Anhydrous iron (iii) chloride may be prepared by union of the elements: Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. To prepare 1000. Laboratory Preparation Of Iron (Iii) Chloride.
From studylib.net
The Synthesis of a Complex Iron Salt Laboratory Preparation Of Iron (Iii) Chloride (i) substance b is anhydrous (fused) calcium chloride (cacl 2). Ferric chloride test is conducted to determine the. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. (ii) b prevents the entry of moisture inside the apparatus because it. (i) substance b is a dehydrating agent. This experiment involves preparing standard solutions of iron (iii) chloride. Laboratory Preparation Of Iron (Iii) Chloride.
From www.studocu.com
Chapter 8 Chapter 8 Study of Compounds A. Hydrogen Chloride Laboratory Preparation Of Iron (Iii) Chloride (ii) anhydrous calciu m chloride absor bs the moisture present in the. (i) substance b is a dehydrating agent. To prepare 1000 ml of a 0.1 mol/l solution of iron (iii) chloride we have to dissolve 27.029 g of fecl3×6h2o (100 % purity) in deionized or. This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and. Laboratory Preparation Of Iron (Iii) Chloride.
From www.researchgate.net
Synthesis illustration, (a) iron rod, (b) scrap iron, (c) solution of Laboratory Preparation Of Iron (Iii) Chloride (ii) anhydrous calciu m chloride absor bs the moisture present in the. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. (i) substance b is a dehydrating agent. (i) substance b is anhydrous (fused) calcium chloride (cacl 2). Anhydrous iron (iii) chloride may be prepared by union of the elements: Ferric chloride test is conducted to. Laboratory Preparation Of Iron (Iii) Chloride.
From classnotes.org.in
Preparation Of Hydrogen Chemistry, Class 11, Hydrogen Laboratory Preparation Of Iron (Iii) Chloride [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). (ii) b prevents the entry of moisture inside the apparatus because it. (i) substance b is a dehydrating agent. This experiment involves preparing standard solutions of iron (iii) chloride at different concentrations and using colorimetric analysis to determine unknown. To prepare 1000 ml of a 0.1. Laboratory Preparation Of Iron (Iii) Chloride.
From www.masterorganicchemistry.com
Reduction of Nitro Groups, The BaeyerVilliger, and Protection of Amines Laboratory Preparation Of Iron (Iii) Chloride Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichlorine. (ii) b prevents the entry of moisture inside the apparatus because it. [2] 2 fe (s) + 3 cl 2 (g) → 2 fecl 3 (s). (i) substance b is a dehydrating agent. Anhydrous iron (iii) chloride may be prepared by union of the elements: (ii) anhydrous. Laboratory Preparation Of Iron (Iii) Chloride.