Electrical Conductors Valence Electrons . this relative mobility of electrons within a material is known as electric conductivity. most atoms hold on to their electrons tightly and are insulators. The atoms of metal elements. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. In copper, the valence electrons are essentially free and strongly. 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged particles. Conductivity is determined by the types of. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two.
from slideplayer.com
— these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged particles. The atoms of metal elements. this relative mobility of electrons within a material is known as electric conductivity. most atoms hold on to their electrons tightly and are insulators. In copper, the valence electrons are essentially free and strongly. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. Conductivity is determined by the types of.
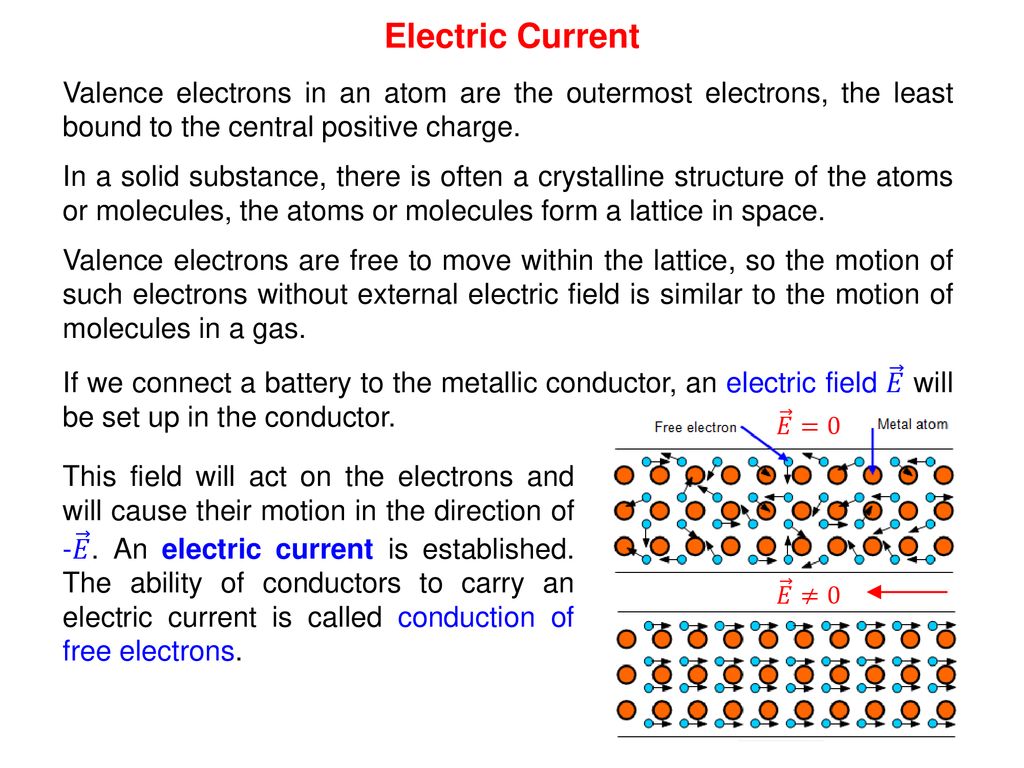
Physics 1 Electric current Ing. Jaroslav Jíra, CSc. ppt download
Electrical Conductors Valence Electrons most atoms hold on to their electrons tightly and are insulators. 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged particles. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. most atoms hold on to their electrons tightly and are insulators. Conductivity is determined by the types of. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. this relative mobility of electrons within a material is known as electric conductivity. The atoms of metal elements. In copper, the valence electrons are essentially free and strongly. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity.
From eepower.com
Understanding the Electrical Properties of Conductors, Insulators, and Electrical Conductors Valence Electrons In copper, the valence electrons are essentially free and strongly. The atoms of metal elements. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two.. Electrical Conductors Valence Electrons.
From slideplayer.com
Static and Dynamic ppt download Electrical Conductors Valence Electrons The atoms of metal elements. most atoms hold on to their electrons tightly and are insulators. 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged particles. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity.. Electrical Conductors Valence Electrons.
From www.youtube.com
Electron Flow in Conductors YouTube Electrical Conductors Valence Electrons Conductivity is determined by the types of. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. In copper, the valence electrons are essentially free and strongly. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. most. Electrical Conductors Valence Electrons.
From www.slideserve.com
PPT Chapter 18 Electrical Properties PowerPoint Presentation, free Electrical Conductors Valence Electrons Conductivity is determined by the types of. The atoms of metal elements. most atoms hold on to their electrons tightly and are insulators. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. this relative mobility of electrons within a material is known as electric. Electrical Conductors Valence Electrons.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID1680708 Electrical Conductors Valence Electrons 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged particles. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. — exciting electrons from the filled valence band to the empty conduction band causes an increase. Electrical Conductors Valence Electrons.
From slideplayer.com
Topics Chapter 2 Voltage, Current and Resistance Atoms ppt download Electrical Conductors Valence Electrons The atoms of metal elements. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. most atoms hold on to their electrons tightly and. Electrical Conductors Valence Electrons.
From roboticsup.com
Valence electrons determine if a material is a conductor or insulator Electrical Conductors Valence Electrons Conductivity is determined by the types of. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. In copper, the valence electrons are essentially free and strongly. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. . Electrical Conductors Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Titanium (Ti)? Electrical Conductors Valence Electrons The atoms of metal elements. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. — these conductors consist of atoms with loosely bound. Electrical Conductors Valence Electrons.
From slidetodoc.com
Basic Electronics I Objectives Define basic components of Electrical Conductors Valence Electrons — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged. Electrical Conductors Valence Electrons.
From slideplayer.com
Chapters 6 & 7 Chemistry 1K Cypress Creek High School ppt download Electrical Conductors Valence Electrons — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. The atoms of metal elements. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. Conductivity is determined by the types of. most atoms hold on to their. Electrical Conductors Valence Electrons.
From slideplayer.com
Voltage, Current, and Resistance ppt download Electrical Conductors Valence Electrons as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. 37 rows — — the electrical conductivity of metals is a result of the movement of electrically. Electrical Conductors Valence Electrons.
From owlcation.com
What Is Electricity? Understanding Volts, Amps, Watts, Ohms, AC and DC Electrical Conductors Valence Electrons — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. The atoms of metal elements. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. In copper, the valence electrons are essentially free and strongly. as you can. Electrical Conductors Valence Electrons.
From studylib.net
PA 81 Electrical conductors and valence electrons Electrical Conductors Valence Electrons The atoms of metal elements. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. In copper, the valence electrons are essentially free and strongly. 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged particles. as you can see, a. Electrical Conductors Valence Electrons.
From slideplayer.com
Atomic Structures Magic of Electrons © 2011 Project Lead The Way, Inc Electrical Conductors Valence Electrons — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. Conductivity is determined by the types of. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. as you can see, a simple count of valence electrons in. Electrical Conductors Valence Electrons.
From slideplayer.com
Section 6.4 “Metallic Bonding” ppt download Electrical Conductors Valence Electrons this relative mobility of electrons within a material is known as electric conductivity. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. The atoms of metal. Electrical Conductors Valence Electrons.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Electrical Conductors Valence Electrons Conductivity is determined by the types of. 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged particles. this relative mobility of electrons within a material is known as electric conductivity. In copper, the valence electrons are essentially free and strongly. as you can see, a simple count of. Electrical Conductors Valence Electrons.
From slideplayer.com
CHAPTER 1 ATOMIC STRUCTURE ppt download Electrical Conductors Valence Electrons as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. most atoms hold on to their electrons tightly and are insulators. — exciting electrons from the. Electrical Conductors Valence Electrons.
From www.expii.com
Valence Electrons — Definition & Importance Expii Electrical Conductors Valence Electrons this relative mobility of electrons within a material is known as electric conductivity. 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged particles. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. Conductivity is determined. Electrical Conductors Valence Electrons.
From slideplayer.com
Topic 6 Fields and Forces ppt download Electrical Conductors Valence Electrons The atoms of metal elements. this relative mobility of electrons within a material is known as electric conductivity. Conductivity is determined by the types of. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. as you can see, a simple count of valence electrons in. Electrical Conductors Valence Electrons.
From themasterchemistry.com
Electrical Conductivities Of Elements In Periodic Table Periodic Electrical Conductors Valence Electrons this relative mobility of electrons within a material is known as electric conductivity. In copper, the valence electrons are essentially free and strongly. Conductivity is determined by the types of. The atoms of metal elements. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. — exciting electrons from. Electrical Conductors Valence Electrons.
From slideplayer.com
The Periodic Table Foldable Notes ppt download Electrical Conductors Valence Electrons as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. 37 rows — — the electrical conductivity of metals is a result of the movement of electrically. Electrical Conductors Valence Electrons.
From slideplayer.com
ELECTRICITY AND ppt download Electrical Conductors Valence Electrons most atoms hold on to their electrons tightly and are insulators. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. The atoms of metal elements. this relative mobility of electrons within a material is known as electric conductivity. as you can see, a simple. Electrical Conductors Valence Electrons.
From www.electrical4u.com
Understanding Valence Electrons and Electrical Conductivity Electrical4U Electrical Conductors Valence Electrons — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. Conductivity is determined by the types of. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. In copper, the valence electrons are essentially free and strongly. this. Electrical Conductors Valence Electrons.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Electrical Conductors Valence Electrons — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. Conductivity is determined by the types of. most atoms hold on to their electrons tightly and are insulators. In copper, the valence electrons are essentially free and strongly. The atoms of metal elements. this relative mobility of electrons within. Electrical Conductors Valence Electrons.
From slideplayer.com
Structure of the Atom & The Periodic Table. ppt download Electrical Conductors Valence Electrons as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. most atoms hold on to their electrons tightly and are insulators. In copper, the valence electrons are essentially free and strongly. Conductivity is determined by the types of. The atoms of metal elements. this relative. Electrical Conductors Valence Electrons.
From www.slideserve.com
PPT Basic Electron Theory PowerPoint Presentation, free download ID Electrical Conductors Valence Electrons as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. this relative mobility of electrons within a material is known as electric conductivity. — these conductors consist of atoms with loosely bound valence electrons that an electric or thermal effect can. 37 rows —. Electrical Conductors Valence Electrons.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Electrical Conductors Valence Electrons 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged particles. most atoms hold on to their electrons tightly and are insulators. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. In copper, the valence electrons. Electrical Conductors Valence Electrons.
From slideplayer.com
Physics 1 Electric current Ing. Jaroslav Jíra, CSc. ppt download Electrical Conductors Valence Electrons most atoms hold on to their electrons tightly and are insulators. 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged particles. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. Conductivity is determined by the types. Electrical Conductors Valence Electrons.
From slideplayer.com
Electrical Theory. ppt download Electrical Conductors Valence Electrons Conductivity is determined by the types of. In copper, the valence electrons are essentially free and strongly. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. The atoms of metal elements. most atoms hold on to their electrons tightly and are insulators. — these conductors. Electrical Conductors Valence Electrons.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Electrical Conductors Valence Electrons — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. The atoms of metal elements. most atoms hold on to their electrons tightly and are insulators. In copper, the valence electrons are essentially free and strongly. 37 rows — — the electrical conductivity of metals is. Electrical Conductors Valence Electrons.
From slideplayer.com
Introduction to Electricity ppt download Electrical Conductors Valence Electrons this relative mobility of electrons within a material is known as electric conductivity. 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged particles. The atoms of metal elements. In copper, the valence electrons are essentially free and strongly. — exciting electrons from the filled valence band to the. Electrical Conductors Valence Electrons.
From www.youtube.com
What is Electric Charge and How Electricity Works Electronics Basics Electrical Conductors Valence Electrons this relative mobility of electrons within a material is known as electric conductivity. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged particles. Conductivity is determined by. Electrical Conductors Valence Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Electrical Conductors Valence Electrons most atoms hold on to their electrons tightly and are insulators. The atoms of metal elements. Conductivity is determined by the types of. In copper, the valence electrons are essentially free and strongly. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. — exciting. Electrical Conductors Valence Electrons.
From electricala2z.com
Difference between Conductor Semiconductor and Insulator Electrical A2Z Electrical Conductors Valence Electrons 37 rows — — the electrical conductivity of metals is a result of the movement of electrically charged particles. — exciting electrons from the filled valence band to the empty conduction band causes an increase in electrical conductivity for two. Conductivity is determined by the types of. In copper, the valence electrons are essentially free and strongly. The. Electrical Conductors Valence Electrons.
From www.alamy.es
Conductor y aislante eléctrico. Diferencia y comparación. Conductor es Electrical Conductors Valence Electrons Conductivity is determined by the types of. as you can see, a simple count of valence electrons in an atom is a poor indicator of a substance’s electrical conductivity. In copper, the valence electrons are essentially free and strongly. most atoms hold on to their electrons tightly and are insulators. — exciting electrons from the filled valence. Electrical Conductors Valence Electrons.