Bromine And Oxygen Formula . When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: Nascent oxygen is a potent oxidizer, capable of. The formula for lithium bromide is \(\ce{libr}\).
from stock.adobe.com
Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. Nascent oxygen is a potent oxidizer, capable of. The formula for lithium bromide is \(\ce{libr}\). Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds.
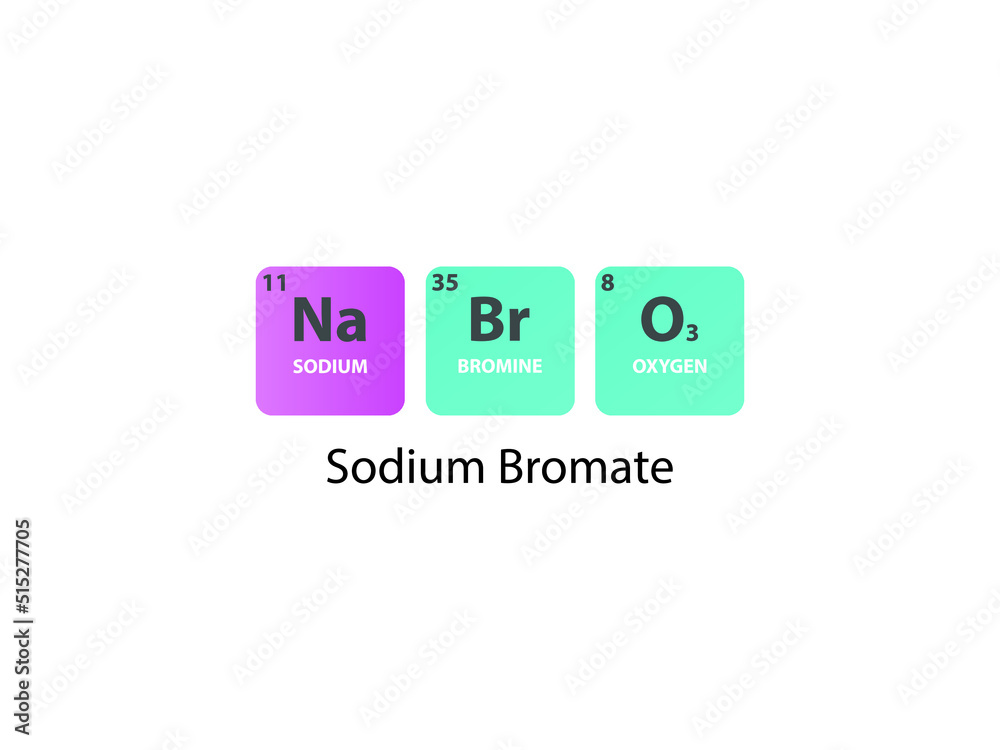
Vecteur Stock NaBrO3 Sodium Bromate molecule. Simple molecular formula
Bromine And Oxygen Formula The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: The formula for lithium bromide is \(\ce{libr}\). Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. Nascent oxygen is a potent oxidizer, capable of. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes.
From www.youtube.com
How to Balance P + Br2 =PBr3 (Phosphorous + Bromine gas) YouTube Bromine And Oxygen Formula The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Nascent oxygen is a potent oxidizer, capable of. Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section. Bromine And Oxygen Formula.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.46 Understand How to Use DotandCross Bromine And Oxygen Formula Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The first ionization energy. Bromine And Oxygen Formula.
From www.numerade.com
SOLVED Draw the condensed structural formulas for all the possible Bromine And Oxygen Formula Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. The formula for lithium bromide is \(\ce{libr}\). The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Nascent oxygen is a potent oxidizer, capable of. Due to its potent oxidatizing action,. Bromine And Oxygen Formula.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Bromine And Oxygen Formula The formula for lithium bromide is \(\ce{libr}\). When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Bromine, br 2, does. Bromine And Oxygen Formula.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine And Oxygen Formula The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2.. Bromine And Oxygen Formula.
From ifunny.co
Scientist Do you know what sodium hydrogen, bromine, and oxygen form Bromine And Oxygen Formula Nascent oxygen is a potent oxidizer, capable of. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. The formula for lithium bromide. Bromine And Oxygen Formula.
From www.chegg.com
Solved A certain compound is made up of one phosphorus (P) Bromine And Oxygen Formula The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. Due to its potent oxidatizing action, bromine. Bromine And Oxygen Formula.
From www.numerade.com
SOLVED Write the chemical formula for this molecule carbon hydrogen Bromine And Oxygen Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Nascent oxygen is a potent oxidizer, capable of. Compounds with the oxidation numbers +1, +3,. Bromine And Oxygen Formula.
From myloview.com
Calculate atomic radius using diatomic molecules oxygen, hydrogen Bromine And Oxygen Formula The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Compounds with the oxidation numbers +1, +3, +4, +5, and +7. Bromine And Oxygen Formula.
From www.pw.live
Bromine Formula, Valency, Mass And Properties Bromine And Oxygen Formula Nascent oxygen is a potent oxidizer, capable of. The formula for lithium bromide is \(\ce{libr}\). Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Due to. Bromine And Oxygen Formula.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine And Oxygen Formula Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. The formula for lithium bromide is \(\ce{libr}\). The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom. Bromine And Oxygen Formula.
From ar.inspiredpencil.com
Bromine Liquid Formula Bromine And Oxygen Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: Due to its potent oxidatizing action, bromine. Bromine And Oxygen Formula.
From www.meritnation.com
Correct formula equation balanced cesium iodide + bromine cesium Bromine And Oxygen Formula The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: Nascent oxygen is a potent oxidizer, capable of. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The formula for lithium bromide is \(\ce{libr}\). Oxyanions are polyatomic ions where oxygen is. Bromine And Oxygen Formula.
From www.researchgate.net
Hydrogen bonds of two independent C5N2H14 2+ cations. Bromine olive Bromine And Oxygen Formula The formula for lithium bromide is \(\ce{libr}\). When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was. Bromine And Oxygen Formula.
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Bromine And Oxygen Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: The first ionization energy. Bromine And Oxygen Formula.
From stock.adobe.com
Vecteur Stock Diatomic molecules diagram shows elements that exist as Bromine And Oxygen Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Nascent oxygen is a potent oxidizer, capable of. The formula for lithium bromide is \(\ce{libr}\).. Bromine And Oxygen Formula.
From stock.adobe.com
Vecteur Stock NaBrO3 Sodium Bromate molecule. Simple molecular formula Bromine And Oxygen Formula Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. The first ionization energy of bromine is. Bromine And Oxygen Formula.
From www.bartleby.com
Answered Write the chemical formula for Bromine… bartleby Bromine And Oxygen Formula Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. The formula for lithium bromide is \(\ce{libr}\). When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and. Bromine And Oxygen Formula.
From mollysrcalderon.blogspot.com
Chemical Formula of Bromide MollysrCalderon Bromine And Oxygen Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. The following equation shows the reaction of hydrogen and oxygen to. Bromine And Oxygen Formula.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID4095104 Bromine And Oxygen Formula Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Nascent oxygen is a potent oxidizer, capable of. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: The first. Bromine And Oxygen Formula.
From www.vecteezy.com
Collection of molecular chemical models combinations from hydrogen Bromine And Oxygen Formula Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Bromine, br 2, does not react with. Bromine And Oxygen Formula.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Bromine And Oxygen Formula The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. The formula for lithium bromide is \(\ce{libr}\). Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Bromine, br 2, does not react with. Bromine And Oxygen Formula.
From www.alamy.com
Bromine molecule Br2 Stock Photo Alamy Bromine And Oxygen Formula Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. The following. Bromine And Oxygen Formula.
From www.alamy.com
Elemental bromine (Br2) molecule. Skeletal formula Stock Vector Image Bromine And Oxygen Formula Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. The formula for lithium bromide is \(\ce{libr}\). The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: Nascent oxygen is a potent oxidizer, capable of. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain. Bromine And Oxygen Formula.
From www.vectorstock.com
Br2 bromine molecule Royalty Free Vector Image Bromine And Oxygen Formula Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. The formula for lithium bromide is \(\ce{libr}\). Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. The first ionization energy of bromine. Bromine And Oxygen Formula.
From www.youtube.com
Write the Formula for Bromine gas YouTube Bromine And Oxygen Formula Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. When an ionic. Bromine And Oxygen Formula.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine And Oxygen Formula Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section. Bromine And Oxygen Formula.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Bromine And Oxygen Formula Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−.. Bromine And Oxygen Formula.
From molekula.com
Purchase Bromine [7726956] online • Catalog • Molekula Group Bromine And Oxygen Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Nascent oxygen is a potent oxidizer, capable of. The following equation shows the reaction of. Bromine And Oxygen Formula.
From myloview.com
Nabro3 sodium bromate molecule. simple molecular formula consisting Bromine And Oxygen Formula The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Oxyanions are polyatomic ions where oxygen is attached to a nonmetal. Bromine And Oxygen Formula.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine And Oxygen Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Bromine, br 2, does not react with oxygen, o 2, or. Bromine And Oxygen Formula.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine And Oxygen Formula Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1,. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: The formula for lithium bromide is \(\ce{libr}\).. Bromine And Oxygen Formula.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Bromine And Oxygen Formula Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine.. Bromine And Oxygen Formula.
From www.chemistrystudent.com
Organic Quick Notes (revision) for A2Level ChemistryStudent Bromine And Oxygen Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. Bromine, br 2, does not react with oxygen, o 2, or nitrogen, n 2. Due to its potent oxidatizing action, bromine. Bromine And Oxygen Formula.
From www.alamy.com
Br2 Stock Vector Images Alamy Bromine And Oxygen Formula When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The formula for lithium bromide is \(\ce{libr}\). Nascent oxygen is a potent oxidizer, capable of. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: Bromine, br 2, does not react with. Bromine And Oxygen Formula.