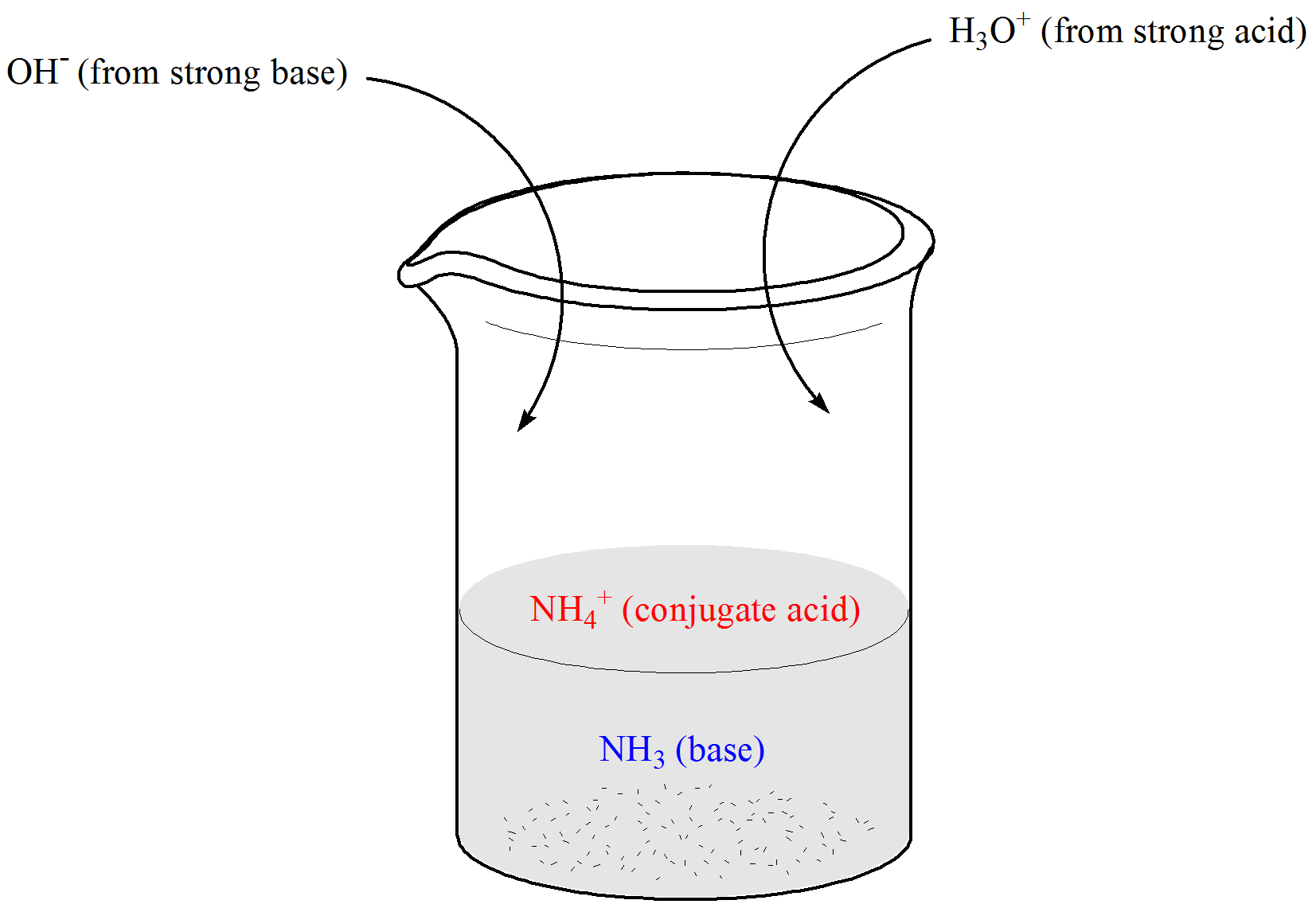
Define Buffer Effect . A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. A buffer is a solution that maintains a constant ph, regardless of the addition of strong acids or strong bases as long. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This study explores the impact of the dilemmas approach, rooted in transformative learning, on enhancing critical thinking skills in. A buffer solution resists a change in ph from the addition of a small amount of acid or base. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial.
from magmastory.blogspot.com
A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer solution resists a change in ph from the addition of a small amount of acid or base. This study explores the impact of the dilemmas approach, rooted in transformative learning, on enhancing critical thinking skills in. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. A buffer is a solution that maintains a constant ph, regardless of the addition of strong acids or strong bases as long.
Which Of The Following Represents A Buffer System? magmastory
Define Buffer Effect A buffer is a solution that maintains a constant ph, regardless of the addition of strong acids or strong bases as long. A buffer is a solution that maintains a constant ph, regardless of the addition of strong acids or strong bases as long. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This study explores the impact of the dilemmas approach, rooted in transformative learning, on enhancing critical thinking skills in. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. It is able to neutralize small.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high Define Buffer Effect Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. A buffer is a solution that maintains a constant ph, regardless of the addition of strong acids or strong bases as long. This characteristic makes buffers important in biological and chemical applications where ph. Define Buffer Effect.
From www.slideserve.com
PPT UNIT V pH, BUFFERS AND ISOTONIC SOLUTION PowerPoint Presentation Define Buffer Effect Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that. Define Buffer Effect.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download Define Buffer Effect A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A solution whose ph is not altered to any great extent by the addition of small quantities of either. Define Buffer Effect.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 Define Buffer Effect A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are characterized by the. Define Buffer Effect.
From www.pinterest.co.uk
Buffer Easy Science Chemistry education, Study chemistry, Chemistry Define Buffer Effect A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. It is able to neutralize small. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers are characterized by the ph range over. Define Buffer Effect.
From slideplayer.com
Buffers A buffer is a mixture of chemicals that make a solution resist Define Buffer Effect It is able to neutralize small. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before.. Define Buffer Effect.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Define Buffer Effect This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph. Define Buffer Effect.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation ID2948821 Define Buffer Effect A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This study explores the impact of the dilemmas approach, rooted in transformative learning, on enhancing critical thinking skills in. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids. Define Buffer Effect.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Define Buffer Effect A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. A buffer solution resists a change in. Define Buffer Effect.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Define Buffer Effect Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A solution whose ph is not altered to any great. Define Buffer Effect.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Define Buffer Effect A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of.. Define Buffer Effect.
From avantecnica.qualitypoolsboulder.com
Buffer Solution Definition, Types, Formula, Examples, and FAQs Define Buffer Effect It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This study explores the impact of the dilemmas approach, rooted in transformative learning,. Define Buffer Effect.
From magmastory.blogspot.com
Which Of The Following Represents A Buffer System? magmastory Define Buffer Effect A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity,. Define Buffer Effect.
From guides.hostos.cuny.edu
Chapter 11 Acids and Bases CHE 110 Introduction to Chemistry Define Buffer Effect A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. This characteristic makes buffers important in biological and chemical applications where ph. Define Buffer Effect.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Define Buffer Effect Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before. This study explores the impact of the dilemmas approach, rooted in transformative learning, on enhancing critical thinking skills in. This characteristic makes buffers important in. Define Buffer Effect.
From jpharmsci.org
Physiological Buffer Effects in Drug Supersaturation A Mechanistic Define Buffer Effect A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and. Define Buffer Effect.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download Define Buffer Effect This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added.. Define Buffer Effect.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Define Buffer Effect A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. It is able to neutralize small. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. A. Define Buffer Effect.
From riaquueta45.blogspot.com
What Is Meant By Buffer Solution Give An Example Of Basic Buffer Define Buffer Effect Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.. Define Buffer Effect.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID720100 Define Buffer Effect A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains a constant ph, regardless of the addition of strong acids or strong. Define Buffer Effect.
From www.researchgate.net
Buffer effect caused by the nanoenvironment of gold NPs in acidic and Define Buffer Effect Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that maintains a constant ph, regardless of. Define Buffer Effect.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Define Buffer Effect This study explores the impact of the dilemmas approach, rooted in transformative learning, on enhancing critical thinking skills in. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that maintains. Define Buffer Effect.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Define Buffer Effect This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer. Define Buffer Effect.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Define Buffer Effect A buffer is a solution that maintains a constant ph, regardless of the addition of strong acids or strong bases as long. This study explores the impact of the dilemmas approach, rooted in transformative learning, on enhancing critical thinking skills in. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and. Define Buffer Effect.
From www.slideshare.net
02 hydrolysis. buffers__colloids Define Buffer Effect It is able to neutralize small. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before. A buffer is a. Define Buffer Effect.
From www.slideshare.net
2012 topic 18 2 buffer solutions Define Buffer Effect A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities. Define Buffer Effect.
From mydigitalkemistry.com
What is a buffer solution simple definition? Free Chemistry Learning Define Buffer Effect A buffer is a solution that maintains a constant ph, regardless of the addition of strong acids or strong bases as long. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. It is able to neutralize small. This study explores the impact. Define Buffer Effect.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Define Buffer Effect Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are. Define Buffer Effect.
From www.slideserve.com
PPT What is a buffer? PowerPoint Presentation, free download ID1149749 Define Buffer Effect A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before. A buffer is a solution that. Define Buffer Effect.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Define Buffer Effect This study explores the impact of the dilemmas approach, rooted in transformative learning, on enhancing critical thinking skills in. A buffer solution resists a change in ph from the addition of a small amount of acid or base. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is a solution that can. Define Buffer Effect.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high Define Buffer Effect A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution resists a change in ph from the addition of a small amount of acid or base. It is able to neutralize small. This characteristic makes buffers important in biological and chemical applications where ph stability. Define Buffer Effect.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions Define Buffer Effect A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. A buffer is a solution that maintains a constant ph, regardless of. Define Buffer Effect.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation Define Buffer Effect Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A solution whose ph is not altered to any great extent by the addition of small quantities of either. Define Buffer Effect.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at Define Buffer Effect A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can. Define Buffer Effect.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation Define Buffer Effect A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. A buffer is a solution. Define Buffer Effect.