Is Nitric Oxide Gas Polar Or Nonpolar . Is nitric oxide polar or nonpolar? Nitric oxide (no) is a weakly polar molecule. Explain how polar compounds differ from nonpolar compounds. Nitric oxide (no) is a polar molecule. Determine if a molecule is polar or nonpolar. Given a pair of compounds, predict. Nitric oxide (no) is a weakly polar molecule. Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. The oxygen atom is more electronegative and strongly attracts the shared electron cloud from the n=o bond. The electronegativity difference between nitrogen. The nitric oxide (no) molecule possesses an identical electron and molecular geometry or shape, i.e., linear. Oxygen (o) atoms are more. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms.
from okgo.net
Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Is nitric oxide polar or nonpolar? Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. Oxygen (o) atoms are more. The oxygen atom is more electronegative and strongly attracts the shared electron cloud from the n=o bond. Nitric oxide (no) is a weakly polar molecule. Nitric oxide (no) is a weakly polar molecule. The electronegativity difference between nitrogen. Given a pair of compounds, predict. Determine if a molecule is polar or nonpolar.
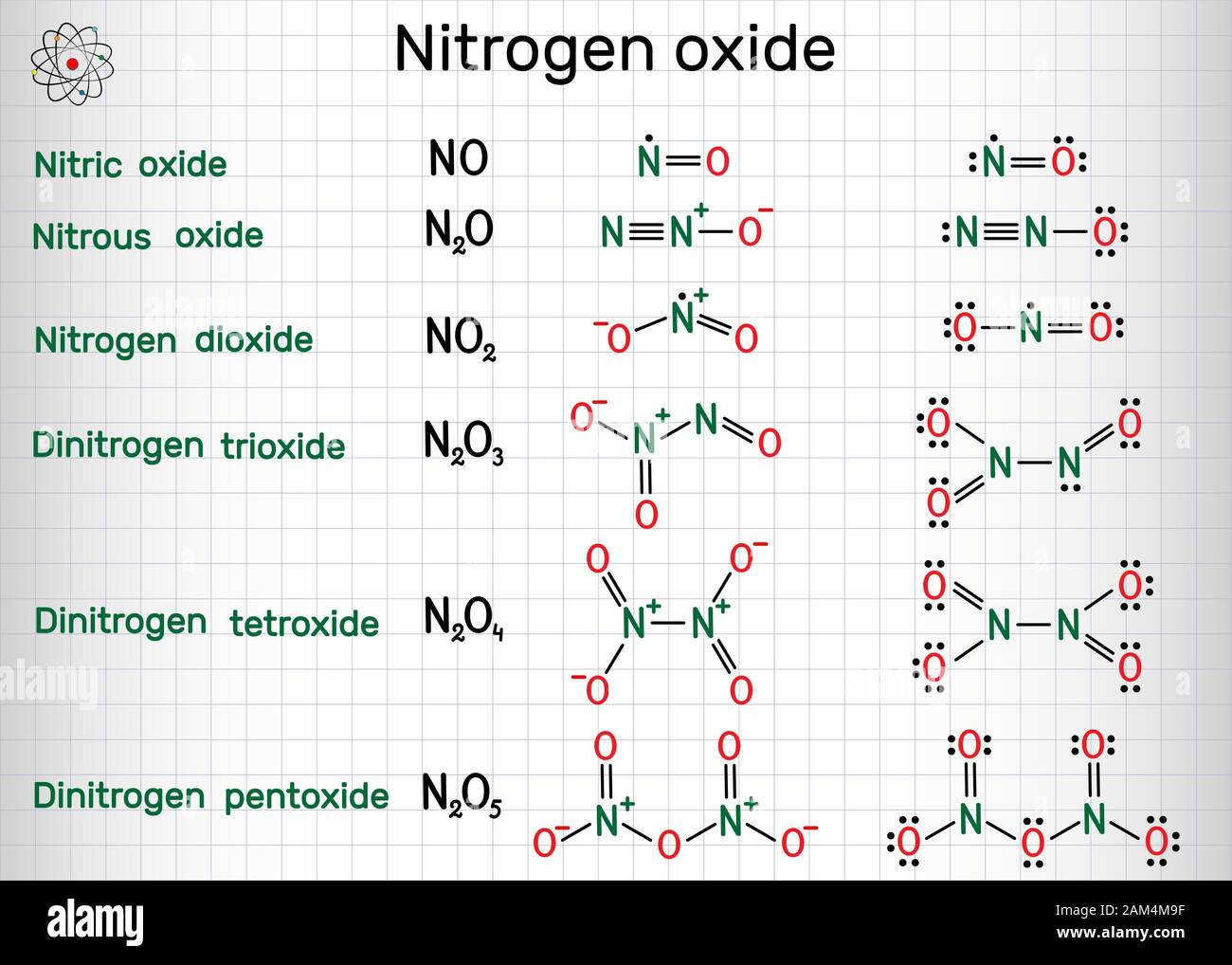
Chemical formulas of nitrogen oxide nitric oxide NO, nitrogen, n2o
Is Nitric Oxide Gas Polar Or Nonpolar Nitric oxide (no) is a weakly polar molecule. Determine if a molecule is polar or nonpolar. Nitric oxide (no) is a weakly polar molecule. Is nitric oxide polar or nonpolar? It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. Nitric oxide (no) is a weakly polar molecule. The electronegativity difference between nitrogen. The nitric oxide (no) molecule possesses an identical electron and molecular geometry or shape, i.e., linear. Given a pair of compounds, predict. Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. The oxygen atom is more electronegative and strongly attracts the shared electron cloud from the n=o bond. Oxygen (o) atoms are more. Nitric oxide (no) is a polar molecule. Explain how polar compounds differ from nonpolar compounds.
From www.slideserve.com
PPT Nitric Oxide PowerPoint Presentation, free download ID4751599 Is Nitric Oxide Gas Polar Or Nonpolar Given a pair of compounds, predict. Determine if a molecule is polar or nonpolar. The electronegativity difference between nitrogen. Oxygen (o) atoms are more. Is nitric oxide polar or nonpolar? Nitric oxide (no) is a weakly polar molecule. It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. The nitric. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.youtube.com
Is N2O Polar or Nonpolar? YouTube Is Nitric Oxide Gas Polar Or Nonpolar Oxygen (o) atoms are more. Determine if a molecule is polar or nonpolar. Given a pair of compounds, predict. Is nitric oxide polar or nonpolar? Explain how polar compounds differ from nonpolar compounds. Nitric oxide (no) is a polar molecule. Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. The electronegativity difference. Is Nitric Oxide Gas Polar Or Nonpolar.
From fphoto.photoshelter.com
nitric oxide gas chemistry vapor Fundamental Photographs The Art of Is Nitric Oxide Gas Polar Or Nonpolar Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Determine if a molecule is polar or nonpolar. Nitric oxide (no) is a polar molecule. Is nitric oxide polar or nonpolar? The oxygen atom is more electronegative and strongly attracts the shared electron cloud from the n=o bond. The. Is Nitric Oxide Gas Polar Or Nonpolar.
From pediaa.com
Difference Between Polar and Nonpolar Molecules Definition, Formation Is Nitric Oxide Gas Polar Or Nonpolar Oxygen (o) atoms are more. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Determine if a molecule is polar or nonpolar. Nitric oxide (no) is a weakly polar molecule. Nitric oxide (no) is a weakly polar molecule. Given a pair of compounds, predict. The electronegativity difference between. Is Nitric Oxide Gas Polar Or Nonpolar.
From present5.com
Biological Effects of Nitric Oxide and its Role Is Nitric Oxide Gas Polar Or Nonpolar Determine if a molecule is polar or nonpolar. Nitric oxide (no) is a polar molecule. Is nitric oxide polar or nonpolar? It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. The oxygen atom is more electronegative and strongly attracts the shared electron cloud from the n=o bond. Nitrogen and. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.youtube.com
HNO3 Polar or Nonpolar (Nitric Acid) YouTube Is Nitric Oxide Gas Polar Or Nonpolar The electronegativity difference between nitrogen. It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. Nitric oxide (no) is a polar molecule. The nitric oxide (no) molecule possesses an identical electron and molecular geometry or shape, i.e., linear. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities,. Is Nitric Oxide Gas Polar Or Nonpolar.
From biologydictionary.net
Paranasal Sinuses The Definitive Guide Biology Dictionary Is Nitric Oxide Gas Polar Or Nonpolar Determine if a molecule is polar or nonpolar. The oxygen atom is more electronegative and strongly attracts the shared electron cloud from the n=o bond. The electronegativity difference between nitrogen. Given a pair of compounds, predict. Explain how polar compounds differ from nonpolar compounds. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table. Is Nitric Oxide Gas Polar Or Nonpolar.
From exyitybtc.blob.core.windows.net
Explain Nitric Oxide at Kimberly Lyons blog Is Nitric Oxide Gas Polar Or Nonpolar Nitric oxide (no) is a polar molecule. Nitric oxide (no) is a weakly polar molecule. The electronegativity difference between nitrogen. Determine if a molecule is polar or nonpolar. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Is nitric oxide polar or nonpolar? It consists of a polar. Is Nitric Oxide Gas Polar Or Nonpolar.
From adelaideghopmullins.blogspot.com
Nh3 Polar or Nonpolar Is Nitric Oxide Gas Polar Or Nonpolar Nitric oxide (no) is a polar molecule. Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. Nitric oxide (no) is a weakly polar molecule. The electronegativity difference between nitrogen. The oxygen atom is more electronegative and strongly attracts the shared electron cloud from the n=o bond. Nitric oxide (no) is a weakly. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.slideserve.com
PPT Nitric Oxide PowerPoint Presentation ID183938 Is Nitric Oxide Gas Polar Or Nonpolar The nitric oxide (no) molecule possesses an identical electron and molecular geometry or shape, i.e., linear. Nitric oxide (no) is a polar molecule. Nitric oxide (no) is a weakly polar molecule. Is nitric oxide polar or nonpolar? It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. The electronegativity difference. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.mitaharaliving.com
Nitric oxide and its potential benefits Is Nitric Oxide Gas Polar Or Nonpolar Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. The electronegativity difference between nitrogen. It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. Oxygen (o) atoms are more. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and. Is Nitric Oxide Gas Polar Or Nonpolar.
From mungfali.com
Nitric Oxide Lewis Structure Is Nitric Oxide Gas Polar Or Nonpolar The electronegativity difference between nitrogen. Nitric oxide (no) is a weakly polar molecule. The nitric oxide (no) molecule possesses an identical electron and molecular geometry or shape, i.e., linear. Determine if a molecule is polar or nonpolar. Given a pair of compounds, predict. Nitric oxide (no) is a weakly polar molecule. Is nitric oxide polar or nonpolar? Oxygen (o) atoms. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.youtube.com
Lewis Structures, Introduction, Formal Charge, Molecular Geometry Is Nitric Oxide Gas Polar Or Nonpolar Nitric oxide (no) is a weakly polar molecule. Determine if a molecule is polar or nonpolar. Nitric oxide (no) is a weakly polar molecule. Explain how polar compounds differ from nonpolar compounds. The electronegativity difference between nitrogen. Oxygen (o) atoms are more. The nitric oxide (no) molecule possesses an identical electron and molecular geometry or shape, i.e., linear. It consists. Is Nitric Oxide Gas Polar Or Nonpolar.
From exypdmjzk.blob.core.windows.net
Nitric Oxide Formula And Structure at Danny Hutchinson blog Is Nitric Oxide Gas Polar Or Nonpolar Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. Nitric oxide (no) is a polar molecule. Is nitric oxide polar or nonpolar? Given a pair of compounds, predict. It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. Nitric oxide (no) is. Is Nitric Oxide Gas Polar Or Nonpolar.
From yesdirt.com
Is N2 Polar or NonPolar? (Nitrogen Gas) Yes Dirt Is Nitric Oxide Gas Polar Or Nonpolar Nitric oxide (no) is a weakly polar molecule. Explain how polar compounds differ from nonpolar compounds. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. Determine if a molecule is polar. Is Nitric Oxide Gas Polar Or Nonpolar.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Oxygen Gas Polar Or Nonpolar Is Nitric Oxide Gas Polar Or Nonpolar The oxygen atom is more electronegative and strongly attracts the shared electron cloud from the n=o bond. Determine if a molecule is polar or nonpolar. Given a pair of compounds, predict. Is nitric oxide polar or nonpolar? Oxygen (o) atoms are more. The nitric oxide (no) molecule possesses an identical electron and molecular geometry or shape, i.e., linear. Nitric oxide. Is Nitric Oxide Gas Polar Or Nonpolar.
From sciencetrends.com
NO Lewis Dot Structure Science Trends Is Nitric Oxide Gas Polar Or Nonpolar The oxygen atom is more electronegative and strongly attracts the shared electron cloud from the n=o bond. Explain how polar compounds differ from nonpolar compounds. Nitric oxide (no) is a polar molecule. Is nitric oxide polar or nonpolar? It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. The nitric. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.researchgate.net
The nitric oxide (NO), nitrite (NO2) and nitrate (NO3) cycle Is Nitric Oxide Gas Polar Or Nonpolar Determine if a molecule is polar or nonpolar. Nitric oxide (no) is a weakly polar molecule. The oxygen atom is more electronegative and strongly attracts the shared electron cloud from the n=o bond. Is nitric oxide polar or nonpolar? Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond.. Is Nitric Oxide Gas Polar Or Nonpolar.
From courses.lumenlearning.com
Covalent Bonds Biology for Majors I Is Nitric Oxide Gas Polar Or Nonpolar Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. The electronegativity difference between nitrogen. Nitric oxide (no) is a weakly polar molecule. Nitric oxide (no) is a weakly polar molecule. The nitric oxide (no) molecule possesses an identical electron and molecular geometry or shape, i.e., linear. Calculate the electronegativity difference (δen) and. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.youtube.com
Is CO2 a Polar or NonPolar Molecule? YouTube Is Nitric Oxide Gas Polar Or Nonpolar The nitric oxide (no) molecule possesses an identical electron and molecular geometry or shape, i.e., linear. Determine if a molecule is polar or nonpolar. Explain how polar compounds differ from nonpolar compounds. The electronegativity difference between nitrogen. Nitric oxide (no) is a weakly polar molecule. Oxygen (o) atoms are more. Nitrogen and oxygen share two electron pairs and form a. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.bigstockphoto.com
Nonpolar Polar Image & Photo (Free Trial) Bigstock Is Nitric Oxide Gas Polar Or Nonpolar Nitric oxide (no) is a weakly polar molecule. Explain how polar compounds differ from nonpolar compounds. Given a pair of compounds, predict. It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. Nitric oxide (no) is a polar molecule. The nitric oxide (no) molecule possesses an identical electron and molecular. Is Nitric Oxide Gas Polar Or Nonpolar.
From pediaa.com
Difference Between Nitric Oxide and Nitrous Oxide Definition Is Nitric Oxide Gas Polar Or Nonpolar Nitric oxide (no) is a weakly polar molecule. Given a pair of compounds, predict. The oxygen atom is more electronegative and strongly attracts the shared electron cloud from the n=o bond. It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. The nitric oxide (no) molecule possesses an identical electron. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.schoenfischlab.com
Nitric oxide SCHOENFISCH LAB Is Nitric Oxide Gas Polar Or Nonpolar The electronegativity difference between nitrogen. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Explain how polar compounds differ from nonpolar compounds. It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. The nitric oxide (no) molecule possesses. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.slideserve.com
PPT Nitric Oxide PowerPoint Presentation ID183938 Is Nitric Oxide Gas Polar Or Nonpolar Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. Determine if a molecule is polar or nonpolar. The oxygen atom is more electronegative and strongly attracts the shared. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.pinterest.com.au
Is NO2+ Polar or Nonpolar? Electron affinity, Covalent bonding Is Nitric Oxide Gas Polar Or Nonpolar Nitric oxide (no) is a weakly polar molecule. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Is nitric oxide polar or nonpolar? Determine if a molecule is polar or nonpolar. The nitric oxide (no) molecule possesses an identical electron and molecular geometry or shape, i.e., linear. Nitrogen. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.youtube.com
Is O2 Polar or Nonpolar? (Oxygen Gas) YouTube Is Nitric Oxide Gas Polar Or Nonpolar It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. Is nitric oxide polar or nonpolar? Oxygen (o) atoms are more. Nitric oxide (no) is a weakly polar molecule. Nitric oxide (no) is a polar molecule. The electronegativity difference between nitrogen. Determine if a molecule is polar or nonpolar. The. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.sciencecoverage.com
Is NO2 Polar or Nonpolar? [Brief Explanation in simple terms] Is Nitric Oxide Gas Polar Or Nonpolar Nitric oxide (no) is a weakly polar molecule. Is nitric oxide polar or nonpolar? Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Given a pair of compounds, predict. The electronegativity difference between nitrogen. Nitrogen and oxygen share two electron pairs and form a double covalent bond in. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.coursehero.com
[Solved] Classify each molecule as polar or nonpolar. Polar Nonpolar Is Nitric Oxide Gas Polar Or Nonpolar The electronegativity difference between nitrogen. Oxygen (o) atoms are more. Determine if a molecule is polar or nonpolar. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Nitric oxide (no) is a weakly polar molecule. Given a pair of compounds, predict. Nitric oxide (no) is a polar molecule.. Is Nitric Oxide Gas Polar Or Nonpolar.
From sciencenotes.org
Polar and Nonpolar Molecules Is Nitric Oxide Gas Polar Or Nonpolar Is nitric oxide polar or nonpolar? Oxygen (o) atoms are more. Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. Determine if a molecule is polar or nonpolar. Nitric oxide (no) is a polar molecule. Nitric oxide (no) is a weakly polar molecule. The electronegativity difference between nitrogen. It consists of a. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.vectorstock.com
Nitric oxide molecule skeletal formula Royalty Free Vector Is Nitric Oxide Gas Polar Or Nonpolar Given a pair of compounds, predict. Explain how polar compounds differ from nonpolar compounds. Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. Oxygen (o) atoms are more. The oxygen atom is. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.youtube.com
Is Nitric oxide (NO) Polar or NonPolar? YouTube Is Nitric Oxide Gas Polar Or Nonpolar Nitric oxide (no) is a weakly polar molecule. It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. Determine if a molecule is polar or nonpolar. Nitric oxide (no) is a polar molecule. Oxygen (o) atoms are more. The oxygen atom is more electronegative and strongly attracts the shared electron. Is Nitric Oxide Gas Polar Or Nonpolar.
From www.numerade.com
SOLVED Complete the following table of greenhouse gases impacting our Is Nitric Oxide Gas Polar Or Nonpolar Is nitric oxide polar or nonpolar? Given a pair of compounds, predict. The nitric oxide (no) molecule possesses an identical electron and molecular geometry or shape, i.e., linear. Nitric oxide (no) is a polar molecule. The oxygen atom is more electronegative and strongly attracts the shared electron cloud from the n=o bond. Determine if a molecule is polar or nonpolar.. Is Nitric Oxide Gas Polar Or Nonpolar.
From cartoondealer.com
Nitric Oxide Formed By The Oxidation Of Nitrogen 3D Illustration Stock Is Nitric Oxide Gas Polar Or Nonpolar Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. Determine if a molecule is polar or nonpolar. Explain how polar compounds differ from nonpolar compounds. Nitric oxide (no) is a weakly polar molecule. It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded. Is Nitric Oxide Gas Polar Or Nonpolar.
From okgo.net
Chemical formulas of nitrogen oxide nitric oxide NO, nitrogen, n2o Is Nitric Oxide Gas Polar Or Nonpolar The nitric oxide (no) molecule possesses an identical electron and molecular geometry or shape, i.e., linear. Nitric oxide (no) is a polar molecule. Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. Is nitric oxide polar or nonpolar? Given a pair of compounds, predict. Oxygen (o) atoms are more. The oxygen atom. Is Nitric Oxide Gas Polar Or Nonpolar.
From fphoto.photoshelter.com
nitric oxide gas chemistry vapor Fundamental Photographs The Art of Is Nitric Oxide Gas Polar Or Nonpolar Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. It consists of a polar n=o double bond due to an electronegativity difference of 0.4 units between the bonded atoms. Oxygen (o) atoms are more. The oxygen atom is more electronegative and strongly attracts the shared electron cloud from. Is Nitric Oxide Gas Polar Or Nonpolar.