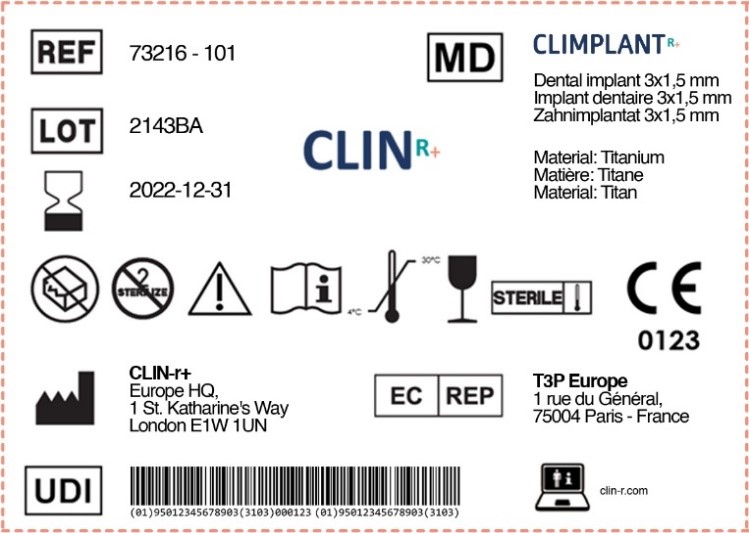
Labelling Requirements For Medical Devices In Europe . Union can give binding interpretations of union law. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. This document aims to provide guidance on different aspects related to standards in the.
from gbu-taganskij.ru
Union can give binding interpretations of union law. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. This document aims to provide guidance on different aspects related to standards in the. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device.
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF
Labelling Requirements For Medical Devices In Europe This document aims to provide guidance on different aspects related to standards in the. This document aims to provide guidance on different aspects related to standards in the. Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. In the european union (eu) they must undergo a conformity. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Union can give binding interpretations of union law. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. Medical devices are products or equipment intended for a medical purpose.
From www.mavenrs.com
Labelling Requirements for Medical Devices in the EU MDR Maven Labelling Requirements For Medical Devices In Europe These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity.. Labelling Requirements For Medical Devices In Europe.
From www.csoftintl.com
THE EU MDR LABELLING JOURNEY BEST PRACTICES FOR NAVIGATING THE LATEST MEDICAL DEVICE LABELLING Labelling Requirements For Medical Devices In Europe Medical devices are products or equipment intended for a medical purpose. This document aims to provide guidance on different aspects related to standards in the. In the european union (eu) they must undergo a conformity. Union can give binding interpretations of union law. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. Labelling Requirements For Medical Devices In Europe.
From www.productcompliancemanager.com
Commission Published Guidance on Medical Devices Labeling Requirements Labelling Requirements For Medical Devices In Europe This document aims to provide guidance on different aspects related to standards in the. Medical devices are products or equipment intended for a medical purpose. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. In the european union (eu) they must undergo a conformity. Compared to the mdd 93/42/eec, the new medical. Labelling Requirements For Medical Devices In Europe.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I more involved Labelling Requirements For Medical Devices In Europe Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Medical devices are products or equipment intended for a medical purpose. These tables aim. Labelling Requirements For Medical Devices In Europe.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labelling Requirements For Medical Devices In Europe The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Union can give binding interpretations of union law. Medical devices are products or equipment intended for a medical purpose. This document aims to provide guidance on different aspects related to standards in the. These tables aim to help. Labelling Requirements For Medical Devices In Europe.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Labelling Requirements For Medical Devices In Europe Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. This document aims to provide guidance on different aspects related to standards in the. Medical devices are products or equipment intended for a medical purpose. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask. Labelling Requirements For Medical Devices In Europe.
From www.scilife.io
Labeling Requirements for Medical Devices Scilife Labelling Requirements For Medical Devices In Europe Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. This document aims to provide guidance on different aspects related to standards in the. Union can give binding interpretations of union law. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds. Labelling Requirements For Medical Devices In Europe.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labelling Requirements For Medical Devices In Europe These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. This document aims to provide guidance on different aspects related to standards in the. Union can give binding interpretations of union law. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the. Labelling Requirements For Medical Devices In Europe.
From www.rimsys.io
CE marking guide for medical devices in the European Union Labelling Requirements For Medical Devices In Europe In the european union (eu) they must undergo a conformity. This document aims to provide guidance on different aspects related to standards in the. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. Union can give binding interpretations of union law. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask. Labelling Requirements For Medical Devices In Europe.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Labelling Requirements For Medical Devices In Europe Union can give binding interpretations of union law. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. This document aims to provide guidance on different aspects related to standards in the. Medical devices are products or equipment intended for a medical purpose. These tables aim to help. Labelling Requirements For Medical Devices In Europe.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labelling Requirements For Medical Devices In Europe These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. In the european union (eu) they must undergo a conformity. This document aims to provide guidance on different aspects. Labelling Requirements For Medical Devices In Europe.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Labelling Requirements For Medical Devices In Europe This document aims to provide guidance on different aspects related to standards in the. Medical devices are products or equipment intended for a medical purpose. Union can give binding interpretations of union law. Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. These tables aim. Labelling Requirements For Medical Devices In Europe.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Labelling Requirements For Medical Devices In Europe In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small.. Labelling Requirements For Medical Devices In Europe.
From mastermindtranslations.co.uk
Language Requirements for Medical Devices in Europe EU MDR Labelling Requirements For Medical Devices In Europe These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. This document aims to provide guidance on different aspects related to standards in the. Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. In the european union (eu). Labelling Requirements For Medical Devices In Europe.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Labelling Requirements For Medical Devices In Europe Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical. Labelling Requirements For Medical Devices In Europe.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Labelling Requirements For Medical Devices In Europe Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. This document aims to provide guidance on different aspects related to standards in the. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. Medical devices are products or. Labelling Requirements For Medical Devices In Europe.
From www.en-standard.eu
BS EN ISO 1522312016 Medical devices. Symbols to be used with medical device labels, labelling Labelling Requirements For Medical Devices In Europe These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. In the european union (eu) they must undergo a conformity. Compared to the mdd 93/42/eec, the new medical devices regulation eu. Labelling Requirements For Medical Devices In Europe.
From blog.clevercompliance.io
EU Medical Device Labelling Requirements Clever Compliance Labelling Requirements For Medical Devices In Europe In the european union (eu) they must undergo a conformity. This document aims to provide guidance on different aspects related to standards in the. Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. These tables aim to help manufacturers of medical devices and in vitro. Labelling Requirements For Medical Devices In Europe.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Labelling Requirements For Medical Devices In Europe The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. In the european union (eu) they must undergo a conformity. This document aims to provide guidance on different aspects related to standards in the. Union can give binding interpretations of union law. Medical devices are products or equipment. Labelling Requirements For Medical Devices In Europe.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Labelling Requirements For Medical Devices In Europe In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. Union can give binding interpretations of union law. This document aims to provide guidance on different aspects related to standards in the. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. Labelling Requirements For Medical Devices In Europe.
From mdlaw.eu
MDR Checklist Labelling & IFU Requirements · MDlaw Information platform on European medical Labelling Requirements For Medical Devices In Europe Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. This document aims to provide guidance on different aspects related to standards in the. Medical devices are products or. Labelling Requirements For Medical Devices In Europe.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Labelling Requirements For Medical Devices In Europe This document aims to provide guidance on different aspects related to standards in the. Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. In the european union (eu) they must undergo a conformity. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for. Labelling Requirements For Medical Devices In Europe.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Labelling Requirements For Medical Devices In Europe The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere. Labelling Requirements For Medical Devices In Europe.
From medicaldevices.freyrsolutions.com
UKCA Marking Requirements for Medical Devices Freyr Medical Devices Labelling Requirements For Medical Devices In Europe In the european union (eu) they must undergo a conformity. This document aims to provide guidance on different aspects related to standards in the. Union can give binding interpretations of union law. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. These tables aim to help manufacturers. Labelling Requirements For Medical Devices In Europe.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Labelling Requirements For Medical Devices In Europe Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to. Labelling Requirements For Medical Devices In Europe.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Registration & Labeling Labelling Requirements For Medical Devices In Europe This document aims to provide guidance on different aspects related to standards in the. Union can give binding interpretations of union law. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. In the european union (eu) they must undergo a conformity. Compared to the mdd 93/42/eec, the new medical devices regulation eu. Labelling Requirements For Medical Devices In Europe.
From kukrejahospital.com
MDR Labelling Requirements in Europe for Medical Devices Labelling Requirements For Medical Devices In Europe Medical devices are products or equipment intended for a medical purpose. Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. This document aims. Labelling Requirements For Medical Devices In Europe.
From www.slideshare.net
MDR Compliance Requirements for Medical Devices in Europe PDF Labelling Requirements For Medical Devices In Europe Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. Union can give binding interpretations of union law. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. In the european union (eu) they. Labelling Requirements For Medical Devices In Europe.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Labelling Requirements For Medical Devices In Europe The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose.. Labelling Requirements For Medical Devices In Europe.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Labelling Requirements For Medical Devices In Europe These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. This document aims to provide guidance on different aspects related to standards in the. In the european union (eu) they must undergo a conformity. Union can give binding interpretations of union law. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask. Labelling Requirements For Medical Devices In Europe.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Labelling Requirements For Medical Devices In Europe Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. In the european union (eu) they must undergo a conformity. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. Medical devices are products or equipment intended for a. Labelling Requirements For Medical Devices In Europe.
From mastermindtranslations.co.uk
EU Update 2024 MDR Language Requirements for Medical Devices Labelling Requirements For Medical Devices In Europe Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a. Labelling Requirements For Medical Devices In Europe.
From gingerproducts.com
Medical device “labelling” language requirements under the EU MDR and IVDR Labelling Requirements For Medical Devices In Europe The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. This document aims to provide guidance on different aspects related to standards in the.. Labelling Requirements For Medical Devices In Europe.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Symbol Use Labelling Requirements For Medical Devices In Europe The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Compared to the mdd 93/42/eec, the new medical devices regulation eu 2017/745 (mdr) sets out additional requirements manufacturers need to adhere to for device. Medical devices are products or equipment intended for a medical purpose. Union can give. Labelling Requirements For Medical Devices In Europe.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labelling Requirements For Medical Devices In Europe The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Union can give binding interpretations of union law. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small. In the european union (eu) they must undergo a conformity. This document aims. Labelling Requirements For Medical Devices In Europe.