Pharmacy Label Legal Requirements . All dispensed medicines are legally required to have a label before being provided to the consumer. Labeling for prescription medicines includes: Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. It sets out the legal framework for labelling and packaging as described in uk legislation. Precautions relating to the use of the medicine: In addition, it describes best. Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. It is a legal requirement for the following to appear on dispensed medicinal products: Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Labeling for patients or caregivers (e.g., medication guides, patient package inserts, and instructions for use).
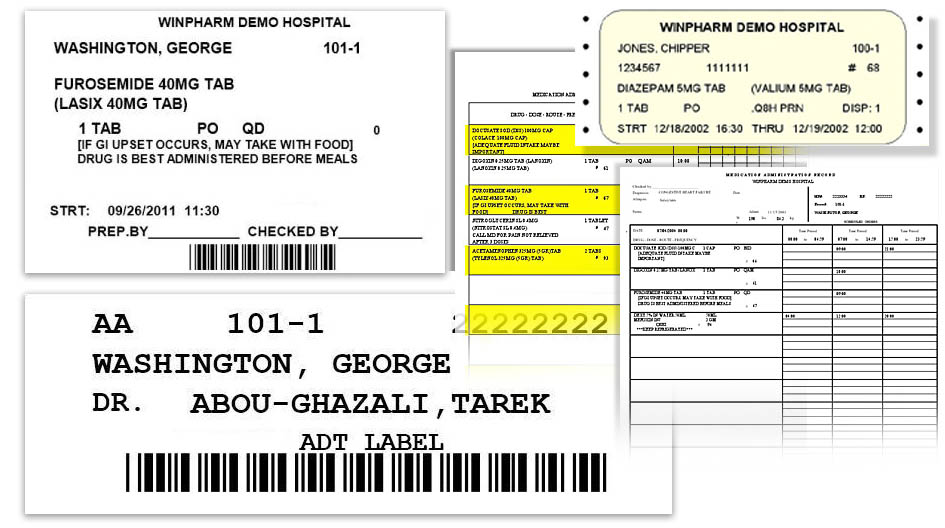
from www.winpharm.com
Labeling for patients or caregivers (e.g., medication guides, patient package inserts, and instructions for use). All dispensed medicines are legally required to have a label before being provided to the consumer. In addition, it describes best. Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. It sets out the legal framework for labelling and packaging as described in uk legislation. It is a legal requirement for the following to appear on dispensed medicinal products: Labeling for prescription medicines includes: Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Precautions relating to the use of the medicine:
WinPharm Hospital Pharmacy Software Sample Labels
Pharmacy Label Legal Requirements Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. In addition, it describes best. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. All dispensed medicines are legally required to have a label before being provided to the consumer. Labeling for prescription medicines includes: Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. It sets out the legal framework for labelling and packaging as described in uk legislation. Labeling for patients or caregivers (e.g., medication guides, patient package inserts, and instructions for use). It is a legal requirement for the following to appear on dispensed medicinal products: Precautions relating to the use of the medicine:
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Pharmacy Label Legal Requirements Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Labeling for patients or caregivers (e.g., medication guides, patient package inserts, and instructions for use). In addition, it describes best. Precautions relating to the use of the medicine: Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing. Pharmacy Label Legal Requirements.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Pharmacy Label Legal Requirements Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Labeling for prescription medicines includes: It is a legal requirement for the following to appear on dispensed medicinal products: In addition, it describes best. Precautions relating. Pharmacy Label Legal Requirements.
From www.nia.nih.gov
Taking Medicines Safely as You Age National Institute on Aging Pharmacy Label Legal Requirements It is a legal requirement for the following to appear on dispensed medicinal products: Labeling for prescription medicines includes: Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. It sets out the legal framework for labelling and packaging as described in uk legislation. Human prescription drug labeling (1). Pharmacy Label Legal Requirements.
From ambitiousmares.blogspot.com
33 Pharmacy Dispensing Label Labels Design Ideas 2020 Pharmacy Label Legal Requirements It sets out the legal framework for labelling and packaging as described in uk legislation. It is a legal requirement for the following to appear on dispensed medicinal products: In addition, it describes best. Labeling for prescription medicines includes: All dispensed medicines are legally required to have a label before being provided to the consumer. Precautions relating to the use. Pharmacy Label Legal Requirements.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Pharmacy Label Legal Requirements Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. Labeling for prescription medicines includes: It sets out the legal framework for labelling and packaging as described in uk legislation. Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Precautions relating to the. Pharmacy Label Legal Requirements.
From www.winpharm.com
WinPharm Hospital Pharmacy Software Sample Labels Pharmacy Label Legal Requirements It sets out the legal framework for labelling and packaging as described in uk legislation. It is a legal requirement for the following to appear on dispensed medicinal products: Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. All dispensed medicines are legally required to have a label before being provided to the consumer.. Pharmacy Label Legal Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Pharmacy Label Legal Requirements All dispensed medicines are legally required to have a label before being provided to the consumer. Labeling for patients or caregivers (e.g., medication guides, patient package inserts, and instructions for use). It is a legal requirement for the following to appear on dispensed medicinal products: Precautions relating to the use of the medicine: Labeling for prescription medicines includes: Chapter (labeling). Pharmacy Label Legal Requirements.
From www.proactpharmacyservices.com
Proact Pharmacy Services Pharmacy Label Legal Requirements Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. It is a legal requirement for the following to appear on dispensed medicinal products: All dispensed medicines are legally required to have a label before being provided to the consumer. Precautions relating to the use of the medicine: Chapter. Pharmacy Label Legal Requirements.
From www.fda.gov.ph
Draft for Comments Guidelines on Labeling Requirements of Drug Pharmacy Label Legal Requirements Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. Precautions relating to the use of the medicine: In addition, it describes best. Labeling for prescription medicines includes: Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. It sets. Pharmacy Label Legal Requirements.
From ar.inspiredpencil.com
Prescription Pharmacy Label Legal Requirements All dispensed medicines are legally required to have a label before being provided to the consumer. In addition, it describes best. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the. Pharmacy Label Legal Requirements.
From www.pfwlabels.com
Pharmacy Label Suppliers Labels for Prescriptions Pharmacy Label Legal Requirements It sets out the legal framework for labelling and packaging as described in uk legislation. All dispensed medicines are legally required to have a label before being provided to the consumer. Labeling for patients or caregivers (e.g., medication guides, patient package inserts, and instructions for use). Human prescription drug labeling (1) contains a summary of the essential scientific information needed. Pharmacy Label Legal Requirements.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Pharmacy Label Legal Requirements Precautions relating to the use of the medicine: Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. It sets out the legal framework for labelling and packaging as described in uk legislation. All dispensed medicines are legally required to have a label before being provided to the consumer. Labels all finished drug products should. Pharmacy Label Legal Requirements.
From www.youtube.com
How to read a medication label YouTube Pharmacy Label Legal Requirements All dispensed medicines are legally required to have a label before being provided to the consumer. It sets out the legal framework for labelling and packaging as described in uk legislation. It is a legal requirement for the following to appear on dispensed medicinal products: Labeling for prescription medicines includes: Labeling for patients or caregivers (e.g., medication guides, patient package. Pharmacy Label Legal Requirements.
From blog.sierralabs.com
5 Best Practices for Pharmaceutical Labeling Pharmacy Label Legal Requirements Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. In addition, it describes best. It is a legal requirement for the following to appear on dispensed medicinal products: All dispensed medicines are legally required to have a label before being provided to the consumer. Labeling for prescription medicines includes: Labeling. Pharmacy Label Legal Requirements.
From www.cutsheetlabels.com
Custom & Templated Pharmacy labels Cut Sheet Labels Pharmacy Label Legal Requirements Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. In addition, it describes best. It is a legal requirement for the following to appear on dispensed medicinal products: Labeling for patients or caregivers (e.g., medication guides, patient package inserts, and instructions for use). Human prescription drug labeling (1). Pharmacy Label Legal Requirements.
From old.sermitsiaq.ag
Medicine Label Template Pharmacy Label Legal Requirements It is a legal requirement for the following to appear on dispensed medicinal products: Labeling for prescription medicines includes: Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Labeling for patients or caregivers. Pharmacy Label Legal Requirements.
From www.icoptix.com
Labeling Laws/FDA and EU Guidance IC Optix Pharmacy Label Legal Requirements Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. All dispensed medicines are legally required to have a label before being provided to the consumer. It is a legal requirement for the following. Pharmacy Label Legal Requirements.
From aplmed.com
4. Documenting Medications (MAR). Aplmed Academy Pharmacy Label Legal Requirements Precautions relating to the use of the medicine: Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. It is a legal requirement for the following to appear on dispensed medicinal products: Labeling for patients or caregivers (e.g., medication guides, patient package inserts, and instructions for use). It sets out the. Pharmacy Label Legal Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Pharmacy Label Legal Requirements Labeling for prescription medicines includes: Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. In addition, it describes best. It sets out the legal framework for labelling and packaging as described in uk. Pharmacy Label Legal Requirements.
From my.clevelandclinic.org
How To Read A Prescription Label A Guide Cleveland Clinic Pharmacy Label Legal Requirements Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Precautions relating to the use of the medicine: All dispensed medicines are legally required to have a label before being provided to the consumer. Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following.. Pharmacy Label Legal Requirements.
From www.slideshare.net
Pharmaceutical labelling Pharmacy Label Legal Requirements Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. It sets out the legal framework for labelling and packaging as described in uk legislation. Labeling for prescription medicines includes: Labeling for patients or caregivers (e.g., medication guides, patient package inserts, and instructions for use). It is a legal requirement for. Pharmacy Label Legal Requirements.
From etactics.com
Prescription Label Design Why It Matters and Effective Examples — Etactics Pharmacy Label Legal Requirements Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Labeling for prescription medicines includes: All dispensed medicines are legally required to have a label before being provided to the consumer. Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Labeling for patients or caregivers. Pharmacy Label Legal Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Pharmacy Label Legal Requirements It sets out the legal framework for labelling and packaging as described in uk legislation. Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. Labeling for patients or caregivers (e.g., medication guides, patient. Pharmacy Label Legal Requirements.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Pharmacy Label Legal Requirements Precautions relating to the use of the medicine: Labeling for prescription medicines includes: Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. It is a legal requirement for the following to appear on dispensed medicinal products: Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at. Pharmacy Label Legal Requirements.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Pharmacy Label Legal Requirements Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Labeling for prescription medicines includes: Labeling for patients or caregivers (e.g., medication guides, patient package inserts, and instructions for use). All dispensed medicines are legally required to have a label before being provided to the consumer. Chapter (labeling) provides definitions and. Pharmacy Label Legal Requirements.
From www.federalregister.gov
Federal Register Requirements on Content and Format of Labeling for Pharmacy Label Legal Requirements In addition, it describes best. Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. Precautions relating to the use of the medicine: Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Labeling for prescription medicines includes: It sets out the legal framework. Pharmacy Label Legal Requirements.
From www.dennybros.com
How to stay compliant when labelling cosmetics Pharmacy Label Legal Requirements Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. It sets out the legal framework for labelling and packaging as described in uk legislation. Precautions relating to the use of the medicine: Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Labeling for patients. Pharmacy Label Legal Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Pharmacy Label Legal Requirements All dispensed medicines are legally required to have a label before being provided to the consumer. It sets out the legal framework for labelling and packaging as described in uk legislation. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Precautions relating to the use of the medicine: In addition,. Pharmacy Label Legal Requirements.
From healthyheels.org
Medication Label Literacy UNC Healthy Heels Pharmacy Label Legal Requirements In addition, it describes best. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following.. Pharmacy Label Legal Requirements.
From www.studocu.com
Law Records & Prescription Requirements RecordKeeping Requirements 1 Pharmacy Label Legal Requirements Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. In addition, it describes best. Precautions relating to the use of the medicine: Labeling for prescription medicines includes: It sets. Pharmacy Label Legal Requirements.
From www.uslegalforms.com
Walmart Pharmacy Form Fill and Sign Printable Template Online US Pharmacy Label Legal Requirements Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Labeling for patients or caregivers (e.g., medication guides, patient package inserts, and instructions for use). Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. It sets out the legal framework for labelling and packaging as. Pharmacy Label Legal Requirements.
From templates.rjuuc.edu.np
Pharmacy Label Template Pharmacy Label Legal Requirements It sets out the legal framework for labelling and packaging as described in uk legislation. Precautions relating to the use of the medicine: In addition, it describes best. Labeling for prescription medicines includes: Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. All dispensed medicines are legally required to have. Pharmacy Label Legal Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Pharmacy Label Legal Requirements Labeling for prescription medicines includes: In addition, it describes best. It is a legal requirement for the following to appear on dispensed medicinal products: Chapter (labeling) provides definitions and labeling standards for official usp articles including strength expression for. Precautions relating to the use of the medicine: Labels all finished drug products should be identified by labelling, as required by. Pharmacy Label Legal Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Pharmacy Label Legal Requirements All dispensed medicines are legally required to have a label before being provided to the consumer. It is a legal requirement for the following to appear on dispensed medicinal products: Labels all finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following. Precautions relating to the use of the medicine: It. Pharmacy Label Legal Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Pharmacy Label Legal Requirements In addition, it describes best. It is a legal requirement for the following to appear on dispensed medicinal products: Precautions relating to the use of the medicine: Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. It sets out the legal framework for labelling and packaging as described in uk. Pharmacy Label Legal Requirements.