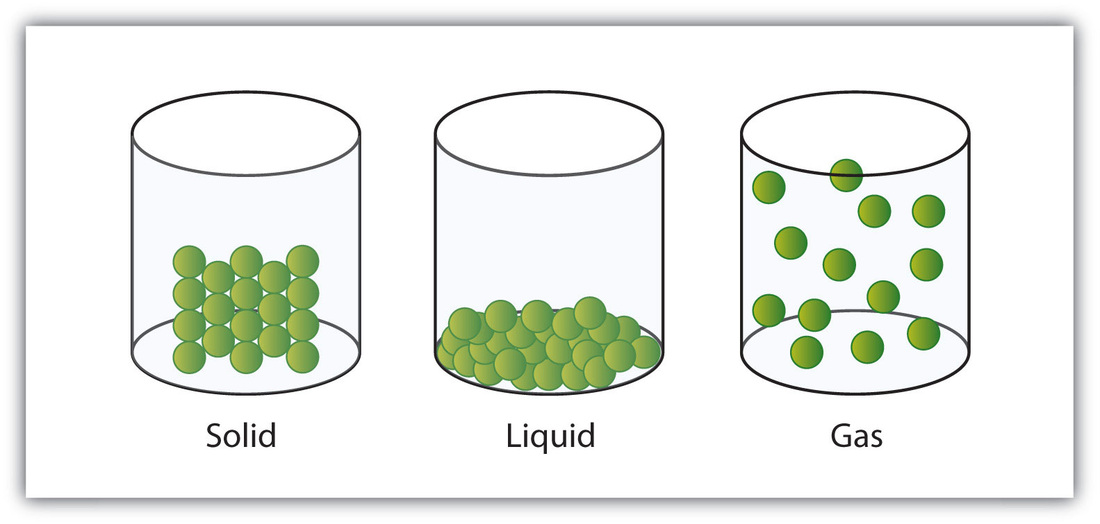
What Is The Particle Energy Level In A Liquid Object . Kinetic theory models the arrangement and movement of particles in solids, liquids and gases. If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. The particle model can be used to explain. As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules. In a system, particles mainly have 2 different types of energy. This model explains the properties of substances in their different. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. The particle model is a model that describes the arrangement and movement of particles in a substance. Kinetic energy allows the particles to move. The different states of matter e.g. The particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. Particles have kinetic (ke) and potential (pe) energy. It explains properties of substances in. Liquids have more kinetic energy than solids. The three states of matter can be represented by the particle model.
from www.oxnotes.com
Particles have kinetic (ke) and potential (pe) energy. This model explains the properties of substances in their different. It explains properties of substances in. The particle model is a model that describes the arrangement and movement of particles in a substance. The particle model can be used to explain. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. In a system, particles mainly have 2 different types of energy. The three states of matter can be represented by the particle model. As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules.
States of Matter Revision Notes IGCSE Chemistry OxNotes GCSE Revision
What Is The Particle Energy Level In A Liquid Object As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules. It explains properties of substances in. In a system, particles mainly have 2 different types of energy. As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules. Particles have kinetic (ke) and potential (pe) energy. The different states of matter e.g. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. The particle model can be used to explain. This model explains the properties of substances in their different. If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. Liquids have more kinetic energy than solids. Kinetic theory models the arrangement and movement of particles in solids, liquids and gases. The particle model is a model that describes the arrangement and movement of particles in a substance. The three states of matter can be represented by the particle model. The particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. Kinetic energy allows the particles to move.
From www.bbc.co.uk
What is the arrangement of particles in a solid, liquid and gas? BBC What Is The Particle Energy Level In A Liquid Object The particle model is a model that describes the arrangement and movement of particles in a substance. As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules. The particle model can be used to explain. The particles of a liquid have enough. What Is The Particle Energy Level In A Liquid Object.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii What Is The Particle Energy Level In A Liquid Object The different states of matter e.g. Particles have kinetic (ke) and potential (pe) energy. If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. Liquids have more kinetic energy than solids. This model explains the properties of substances in their different. The. What Is The Particle Energy Level In A Liquid Object.
From hubpages.com
Atoms and Atomic Structure HubPages What Is The Particle Energy Level In A Liquid Object If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. Liquids have more kinetic energy than solids. The particle model is a model that describes the arrangement and movement of particles in a substance. The different states of matter e.g. The particle. What Is The Particle Energy Level In A Liquid Object.
From studyrocket.co.uk
The Particle Model GCSE Chemistry Science) OCR Revision What Is The Particle Energy Level In A Liquid Object Kinetic theory models the arrangement and movement of particles in solids, liquids and gases. The particle model can be used to explain. The particle model is a model that describes the arrangement and movement of particles in a substance. The three states of matter can be represented by the particle model. If you heat a liquid strongly enough, eventually the. What Is The Particle Energy Level In A Liquid Object.
From www.expii.com
What Are the Phases of Matter? — Overview & Examples Expii What Is The Particle Energy Level In A Liquid Object In a system, particles mainly have 2 different types of energy. As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules. Kinetic energy allows the particles to move. If you heat a liquid strongly enough, eventually the particles will be moving around. What Is The Particle Energy Level In A Liquid Object.
From slideplayer.com
States of Matter States of Matter. ppt download What Is The Particle Energy Level In A Liquid Object It explains properties of substances in. Liquids have more kinetic energy than solids. Particles have kinetic (ke) and potential (pe) energy. As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules. The particle model is a model that describes the arrangement and. What Is The Particle Energy Level In A Liquid Object.
From www.teachoo.com
What are the characteristics of the particles of matter? Teachoo What Is The Particle Energy Level In A Liquid Object The particle model is a model that describes the arrangement and movement of particles in a substance. The three states of matter can be represented by the particle model. If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. In a system,. What Is The Particle Energy Level In A Liquid Object.
From www.goodscience.com.au
Particles in Solids, Liquids and Gases Good Science What Is The Particle Energy Level In A Liquid Object If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules. If you heat a liquid strongly enough, eventually the particles will. What Is The Particle Energy Level In A Liquid Object.
From guidedehartsicklewort.z21.web.core.windows.net
Particle Diagram Solid What Is The Particle Energy Level In A Liquid Object Liquids have more kinetic energy than solids. The different states of matter e.g. The particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. The particle model can be used to explain. Kinetic energy allows the particles to move. Particles have kinetic (ke) and potential (pe) energy. As for gases,. What Is The Particle Energy Level In A Liquid Object.
From www.slideserve.com
PPT Chapter 2 A Particle View of Matter PowerPoint Presentation What Is The Particle Energy Level In A Liquid Object Kinetic energy allows the particles to move. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. The particle model is. What Is The Particle Energy Level In A Liquid Object.
From www.slideserve.com
PPT Energy and States of Matter PowerPoint Presentation, free What Is The Particle Energy Level In A Liquid Object The three states of matter can be represented by the particle model. The different states of matter e.g. Kinetic energy allows the particles to move. Liquids have more kinetic energy than solids. This model explains the properties of substances in their different. As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid. What Is The Particle Energy Level In A Liquid Object.
From www.oxnotes.com
States of Matter Revision Notes IGCSE Chemistry OxNotes GCSE Revision What Is The Particle Energy Level In A Liquid Object If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. Particles have kinetic (ke) and potential (pe) energy. The different states of matter e.g. In a system, particles mainly have 2 different types of energy. The particle model can be used to. What Is The Particle Energy Level In A Liquid Object.
From www.dreamstime.com
Density of Matter with Gas, Liquid and Solid Particle States Outline What Is The Particle Energy Level In A Liquid Object In a system, particles mainly have 2 different types of energy. The different states of matter e.g. Particles have kinetic (ke) and potential (pe) energy. The three states of matter can be represented by the particle model. The particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. If you. What Is The Particle Energy Level In A Liquid Object.
From owlcation.com
What Is the Particle Model? A Guide to Solids, Liquids and Gases What Is The Particle Energy Level In A Liquid Object Kinetic theory models the arrangement and movement of particles in solids, liquids and gases. The three states of matter can be represented by the particle model. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. In a system, particles mainly have 2 different types of energy. Kinetic energy. What Is The Particle Energy Level In A Liquid Object.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement What Is The Particle Energy Level In A Liquid Object Kinetic energy allows the particles to move. The particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules. In a system, particles. What Is The Particle Energy Level In A Liquid Object.
From chemstuff.co.uk
Particle Model of Solids, Liquids and Gases Chemstuff What Is The Particle Energy Level In A Liquid Object In a system, particles mainly have 2 different types of energy. The particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. The three states of matter can be represented by the particle model. Kinetic energy allows the particles to move. Kinetic theory models the arrangement and movement of particles. What Is The Particle Energy Level In A Liquid Object.
From owlcation.com
What Is the Particle Model? A Guide to Solids, Liquids and Gases What Is The Particle Energy Level In A Liquid Object The particle model can be used to explain. Liquids have more kinetic energy than solids. This model explains the properties of substances in their different. If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. Particles have kinetic (ke) and potential (pe). What Is The Particle Energy Level In A Liquid Object.
From www.slideserve.com
PPT The Nature of Matter PowerPoint Presentation, free download ID What Is The Particle Energy Level In A Liquid Object In a system, particles mainly have 2 different types of energy. Liquids have more kinetic energy than solids. The three states of matter can be represented by the particle model. The particle model can be used to explain. This model explains the properties of substances in their different. The different states of matter e.g. Particles have kinetic (ke) and potential. What Is The Particle Energy Level In A Liquid Object.
From gioujmagc.blob.core.windows.net
Particles In Solids Liquids And Gases Bbc Bitesize at Julian Pryor blog What Is The Particle Energy Level In A Liquid Object The particle model can be used to explain. It explains properties of substances in. If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. Particles have kinetic (ke) and potential (pe) energy. The particle model is a model that describes the arrangement. What Is The Particle Energy Level In A Liquid Object.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in What Is The Particle Energy Level In A Liquid Object Kinetic energy allows the particles to move. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. The particle model is a model that describes the arrangement and movement of particles in a substance. The three states of matter can be represented by the particle model. If you heat. What Is The Particle Energy Level In A Liquid Object.
From johnwest.edublogs.org
Solids liquids and gasses, a story in three parts. Science Connected What Is The Particle Energy Level In A Liquid Object The particle model is a model that describes the arrangement and movement of particles in a substance. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. In a system, particles mainly have 2 different types of energy. Liquids have more kinetic energy than solids. If you heat a. What Is The Particle Energy Level In A Liquid Object.
From sciencenotes.org
States of Matter What Is The Particle Energy Level In A Liquid Object In a system, particles mainly have 2 different types of energy. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules.. What Is The Particle Energy Level In A Liquid Object.
From www.teachoo.com
Characterstics of Particles of Matter Class 9 Science Notes Teacho What Is The Particle Energy Level In A Liquid Object Kinetic energy allows the particles to move. Particles have kinetic (ke) and potential (pe) energy. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. The particle model can be used to explain. As for gases, increasing the temperature increases both the average kinetic energy of the particles in. What Is The Particle Energy Level In A Liquid Object.
From grigerscience.weebly.com
Properties of Matter Griger Science What Is The Particle Energy Level In A Liquid Object Kinetic energy allows the particles to move. The particle model can be used to explain. The particle model is a model that describes the arrangement and movement of particles in a substance. As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules.. What Is The Particle Energy Level In A Liquid Object.
From online-learning-college.com
The particle model What is the model? Solids, Liquids & Gases What Is The Particle Energy Level In A Liquid Object If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. The three states of matter can be represented by the particle model. The particle model can be used to explain. Liquids have more kinetic energy than solids. Particles have kinetic (ke) and. What Is The Particle Energy Level In A Liquid Object.
From www.slideserve.com
PPT CHEMISTRY XL14A NATURE OF LIGHT AND THE ATOM PowerPoint What Is The Particle Energy Level In A Liquid Object The particle model is a model that describes the arrangement and movement of particles in a substance. If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. Particles have kinetic (ke) and potential (pe) energy. Kinetic energy allows the particles to move.. What Is The Particle Energy Level In A Liquid Object.
From sciencetallis.weebly.com
3. Particle Model of Matter THOMAS TALLIS SCIENCE What Is The Particle Energy Level In A Liquid Object The particle model is a model that describes the arrangement and movement of particles in a substance. As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules. Kinetic theory models the arrangement and movement of particles in solids, liquids and gases. In. What Is The Particle Energy Level In A Liquid Object.
From www.slideserve.com
PPT Material since exam 3 PowerPoint Presentation, free download ID What Is The Particle Energy Level In A Liquid Object As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules. The particle model is a model that describes the arrangement and movement of particles in a substance. Kinetic theory models the arrangement and movement of particles in solids, liquids and gases. Kinetic. What Is The Particle Energy Level In A Liquid Object.
From www.snexplores.org
Explainer What are the different states of matter? What Is The Particle Energy Level In A Liquid Object The particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. The particle model is a model that describes the arrangement and movement of particles in a substance. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. This. What Is The Particle Energy Level In A Liquid Object.
From www.slideserve.com
PPT Ch 5 Atomic Structure and the Periodic Table PowerPoint What Is The Particle Energy Level In A Liquid Object The three states of matter can be represented by the particle model. It explains properties of substances in. In a system, particles mainly have 2 different types of energy. Kinetic theory models the arrangement and movement of particles in solids, liquids and gases. Kinetic energy allows the particles to move. The different states of matter e.g. The particle model can. What Is The Particle Energy Level In A Liquid Object.
From schematicviciosinfin17.z22.web.core.windows.net
Solid Liquid And Gas Particle Diagram What Is The Particle Energy Level In A Liquid Object If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. Particles have kinetic (ke) and potential (pe) energy. Kinetic theory models. What Is The Particle Energy Level In A Liquid Object.
From www.slideshare.net
Particle Theory (Slg Introduction) What Is The Particle Energy Level In A Liquid Object This model explains the properties of substances in their different. The different states of matter e.g. Kinetic theory models the arrangement and movement of particles in solids, liquids and gases. As for gases, increasing the temperature increases both the average kinetic energy of the particles in a liquid and the range of kinetic energy of the individual molecules. Particles have. What Is The Particle Energy Level In A Liquid Object.
From www.slideserve.com
PPT States of Matter A. The Theory PowerPoint Presentation What Is The Particle Energy Level In A Liquid Object If you heat a liquid strongly enough, eventually the particles will be moving around with enough energy that they overcome the forces holding them together in the liquid. The particle model can be used to explain. In a system, particles mainly have 2 different types of energy. As for gases, increasing the temperature increases both the average kinetic energy of. What Is The Particle Energy Level In A Liquid Object.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning What Is The Particle Energy Level In A Liquid Object The particle model can be used to explain. The particles of a liquid have enough energy to break free of some of the forces of attraction between the particles. Particles have kinetic (ke) and potential (pe) energy. Kinetic energy allows the particles to move. In a system, particles mainly have 2 different types of energy. The three states of matter. What Is The Particle Energy Level In A Liquid Object.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii What Is The Particle Energy Level In A Liquid Object Particles have kinetic (ke) and potential (pe) energy. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. The different states of matter e.g. This model explains the properties of substances in their different. Kinetic energy allows the particles to move. The three states of matter can be represented. What Is The Particle Energy Level In A Liquid Object.