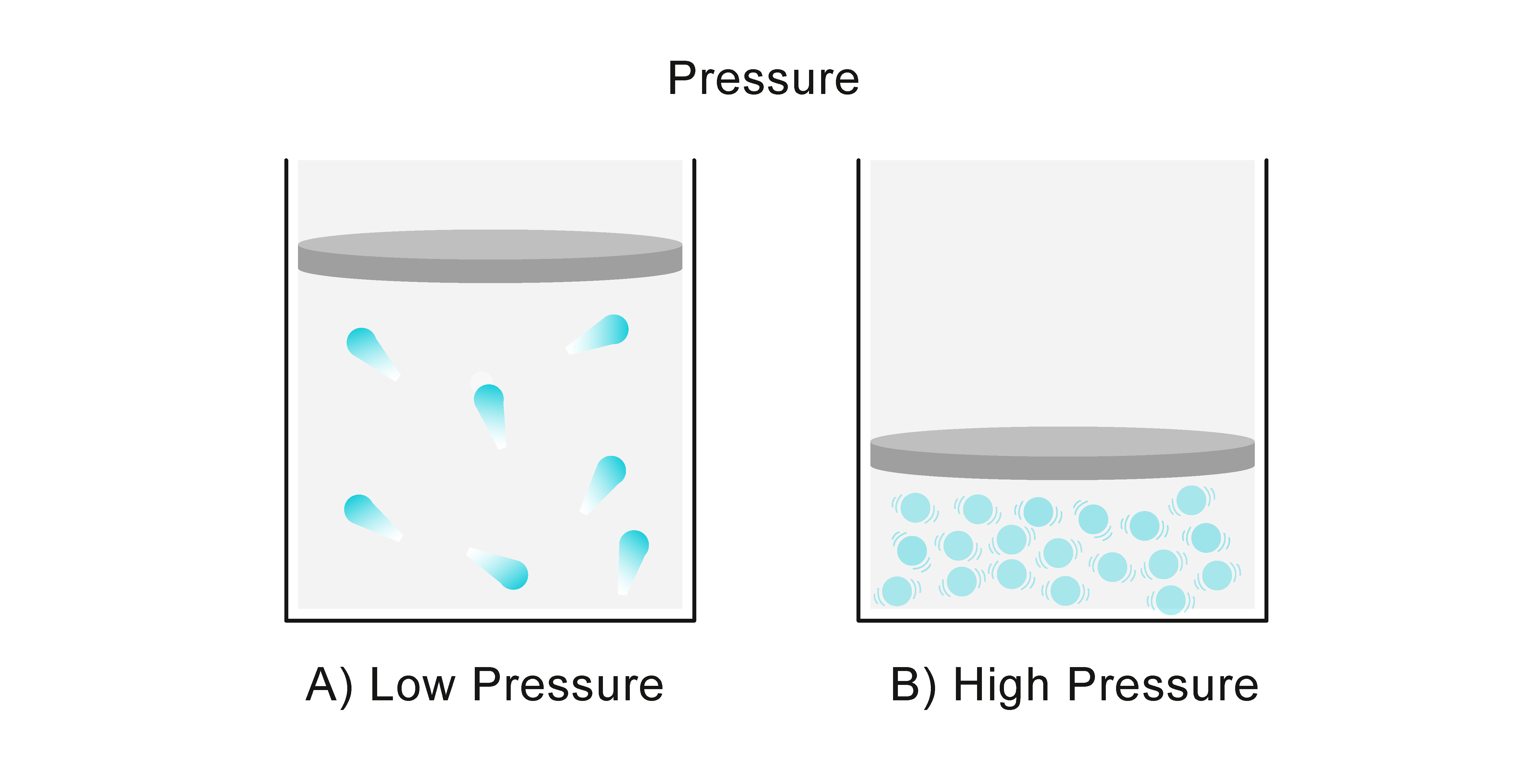
How Do You Calculate The Volume Of A Gas Under Pressure . P1 × v1 = p2 × v2. We can write the boyle's law equation in the following way: volume of a gas sample the sample of gas in figure 9.13 has a volume of 15.0 ml at a pressure of 13.0 psi. boyle's law formula. this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,. Where p1 and v1 are initial pressure and. if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount. it is summarized in the statement now known as boyle’s law: if you take the pressure value and multiply it by the volume value, the product is a constant (k) for a given. The volume of a given amount of gas held at constant temperature is inversely proportional to. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature.
from www.edplace.com
We can write the boyle's law equation in the following way: boyle's law formula. The volume of a given amount of gas held at constant temperature is inversely proportional to. it is summarized in the statement now known as boyle’s law: this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,. if you take the pressure value and multiply it by the volume value, the product is a constant (k) for a given. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. Where p1 and v1 are initial pressure and. if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount. P1 × v1 = p2 × v2.
Understand Gas Pressure Worksheet EdPlace
How Do You Calculate The Volume Of A Gas Under Pressure you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. boyle's law formula. The volume of a given amount of gas held at constant temperature is inversely proportional to. it is summarized in the statement now known as boyle’s law: you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. We can write the boyle's law equation in the following way: P1 × v1 = p2 × v2. if you take the pressure value and multiply it by the volume value, the product is a constant (k) for a given. if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount. Where p1 and v1 are initial pressure and. volume of a gas sample the sample of gas in figure 9.13 has a volume of 15.0 ml at a pressure of 13.0 psi. this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,.
From loankohnert1957.blogspot.com
Loan Kohnert How Do You Find The Volume Of A Gas Given Temperature And How Do You Calculate The Volume Of A Gas Under Pressure Where p1 and v1 are initial pressure and. We can write the boyle's law equation in the following way: if you take the pressure value and multiply it by the volume value, the product is a constant (k) for a given. this ideal gas law calculator will help you establish the properties of an ideal gas subject to. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.visionlearning.com
Properties of Gases Chemistry Visionlearning How Do You Calculate The Volume Of A Gas Under Pressure it is summarized in the statement now known as boyle’s law: P1 × v1 = p2 × v2. volume of a gas sample the sample of gas in figure 9.13 has a volume of 15.0 ml at a pressure of 13.0 psi. this ideal gas law calculator will help you establish the properties of an ideal gas. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.tec-science.com
Specific heat capacity of gases (at constant volume or pressure) tec How Do You Calculate The Volume Of A Gas Under Pressure boyle's law formula. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. Where p1 and v1 are initial pressure and. this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,. it is summarized in the statement. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.edplace.com
Understand Gas Pressure Worksheet EdPlace How Do You Calculate The Volume Of A Gas Under Pressure this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,. if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount. volume of a gas sample the sample of gas in figure 9.13 has a volume of. How Do You Calculate The Volume Of A Gas Under Pressure.
From masterconceptsinchemistry.com
How to find mass of gas given volume, temperature and pressure in the How Do You Calculate The Volume Of A Gas Under Pressure if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount. Where p1 and v1 are initial pressure and. We can write the boyle's law equation in the following way: you can use the ideal gas volume calculator to find the molar volume of an ideal gas. How Do You Calculate The Volume Of A Gas Under Pressure.
From chem.libretexts.org
6.3 Relationships among Pressure, Temperature, Volume, and Amount How Do You Calculate The Volume Of A Gas Under Pressure you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. P1 × v1 = p2 × v2. We can write the boyle's law equation in the following way: boyle's law formula. Where p1 and v1 are initial pressure and. if you take the pressure value and multiply. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.grc.nasa.gov
Work Done by a Gas How Do You Calculate The Volume Of A Gas Under Pressure if you take the pressure value and multiply it by the volume value, the product is a constant (k) for a given. this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,. volume of a gas sample the sample of gas in figure 9.13 has a volume of. How Do You Calculate The Volume Of A Gas Under Pressure.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas How Do You Calculate The Volume Of A Gas Under Pressure P1 × v1 = p2 × v2. if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount. it is summarized in the statement now known as boyle’s law: you can use the ideal gas volume calculator to find the molar volume of an ideal gas. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock How Do You Calculate The Volume Of A Gas Under Pressure if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount. P1 × v1 = p2 × v2. volume of a gas sample the sample of gas in figure 9.13 has a volume of 15.0 ml at a pressure of 13.0 psi. The volume of a given. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.youtube.com
Calculating Gas Pressure Pressure in the Gas Laws CLEAR & SIMPLE How Do You Calculate The Volume Of A Gas Under Pressure it is summarized in the statement now known as boyle’s law: boyle's law formula. The volume of a given amount of gas held at constant temperature is inversely proportional to. Where p1 and v1 are initial pressure and. We can write the boyle's law equation in the following way: if you take the pressure value and multiply. How Do You Calculate The Volume Of A Gas Under Pressure.
From masterconceptsinchemistry.com
As pressure increase the volume of gas decrease How Do You Calculate The Volume Of A Gas Under Pressure volume of a gas sample the sample of gas in figure 9.13 has a volume of 15.0 ml at a pressure of 13.0 psi. if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount. We can write the boyle's law equation in the following way: . How Do You Calculate The Volume Of A Gas Under Pressure.
From www.coursehero.com
[Solved] Using the ideal gas equation calculate the volume that 15 g of How Do You Calculate The Volume Of A Gas Under Pressure this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,. We can write the boyle's law equation in the following way: The volume of a given amount of gas held at constant temperature is inversely proportional to. you can use the ideal gas volume calculator to find the molar. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii How Do You Calculate The Volume Of A Gas Under Pressure this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,. Where p1 and v1 are initial pressure and. The volume of a given amount of gas held at constant temperature is inversely proportional to. if you take the pressure value and multiply it by the volume value, the product. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint How Do You Calculate The Volume Of A Gas Under Pressure it is summarized in the statement now known as boyle’s law: boyle's law formula. if you take the pressure value and multiply it by the volume value, the product is a constant (k) for a given. this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,. . How Do You Calculate The Volume Of A Gas Under Pressure.
From www.youtube.com
Volume of 1 mole of any gas at STP using the ideal gas equation YouTube How Do You Calculate The Volume Of A Gas Under Pressure it is summarized in the statement now known as boyle’s law: P1 × v1 = p2 × v2. boyle's law formula. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. Where p1 and v1 are initial pressure and. if you take the pressure value and. How Do You Calculate The Volume Of A Gas Under Pressure.
From mungfali.com
Boyle's Law Chart How Do You Calculate The Volume Of A Gas Under Pressure if you take the pressure value and multiply it by the volume value, the product is a constant (k) for a given. this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,. We can write the boyle's law equation in the following way: you can use the ideal. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.youtube.com
Gas Pressure and Volume GCSE Physics Revision YouTube How Do You Calculate The Volume Of A Gas Under Pressure boyle's law formula. if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount. The volume of a given amount of gas held at constant temperature is inversely proportional to. if you take the pressure value and multiply it by the volume value, the product is. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint How Do You Calculate The Volume Of A Gas Under Pressure if you take the pressure value and multiply it by the volume value, the product is a constant (k) for a given. Where p1 and v1 are initial pressure and. if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount. P1 × v1 = p2 ×. How Do You Calculate The Volume Of A Gas Under Pressure.
From dxokdgtal.blob.core.windows.net
How Do You Calculate Moles Of Gas at Bobby Andersen blog How Do You Calculate The Volume Of A Gas Under Pressure it is summarized in the statement now known as boyle’s law: Where p1 and v1 are initial pressure and. if you take the pressure value and multiply it by the volume value, the product is a constant (k) for a given. The volume of a given amount of gas held at constant temperature is inversely proportional to. . How Do You Calculate The Volume Of A Gas Under Pressure.
From www.grc.nasa.gov
Entropy of a Gas How Do You Calculate The Volume Of A Gas Under Pressure P1 × v1 = p2 × v2. Where p1 and v1 are initial pressure and. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. boyle's law formula. The volume of a given amount of gas held at constant temperature is inversely proportional to. volume of a. How Do You Calculate The Volume Of A Gas Under Pressure.
From en.ppt-online.org
The ideal gas equation online presentation How Do You Calculate The Volume Of A Gas Under Pressure volume of a gas sample the sample of gas in figure 9.13 has a volume of 15.0 ml at a pressure of 13.0 psi. Where p1 and v1 are initial pressure and. boyle's law formula. if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount.. How Do You Calculate The Volume Of A Gas Under Pressure.
From studylib.net
The Molar Volume of a Gas How Do You Calculate The Volume Of A Gas Under Pressure boyle's law formula. Where p1 and v1 are initial pressure and. it is summarized in the statement now known as boyle’s law: if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount. We can write the boyle's law equation in the following way: this. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.nagwa.com
Question Video Calculating the Change of the Volume of a Gas under How Do You Calculate The Volume Of A Gas Under Pressure you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. volume of a gas sample the sample of gas in figure 9.13 has a volume of 15.0 ml at a pressure of 13.0 psi. We can write the boyle's law equation in the following way: The volume of. How Do You Calculate The Volume Of A Gas Under Pressure.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law How Do You Calculate The Volume Of A Gas Under Pressure if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount. Where p1 and v1 are initial pressure and. The volume of a given amount of gas held at constant temperature is inversely proportional to. P1 × v1 = p2 × v2. you can use the ideal. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4144868 How Do You Calculate The Volume Of A Gas Under Pressure boyle's law formula. it is summarized in the statement now known as boyle’s law: this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,. The volume of a given amount of gas held at constant temperature is inversely proportional to. Where p1 and v1 are initial pressure and.. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.nagwa.com
Question Video Calculating Gas Volume after It Is Heated at Constant How Do You Calculate The Volume Of A Gas Under Pressure if you take the pressure value and multiply it by the volume value, the product is a constant (k) for a given. The volume of a given amount of gas held at constant temperature is inversely proportional to. P1 × v1 = p2 × v2. Where p1 and v1 are initial pressure and. this ideal gas law calculator. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.slideserve.com
PPT Chapter 14 Properties of Gases PowerPoint Presentation, free How Do You Calculate The Volume Of A Gas Under Pressure this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. boyle's law formula. We can write the boyle's law equation in the following way: it is summarized. How Do You Calculate The Volume Of A Gas Under Pressure.
From socratic.org
A sample of a gas has a volume of 2.0 liters at a pressure of 1.0 How Do You Calculate The Volume Of A Gas Under Pressure The volume of a given amount of gas held at constant temperature is inversely proportional to. We can write the boyle's law equation in the following way: you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. P1 × v1 = p2 × v2. volume of a gas. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.youtube.com
11.8 The Ideal Gas Law Pressure, Volume, Temperature, & Moles YouTube How Do You Calculate The Volume Of A Gas Under Pressure boyle's law formula. this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,. The volume of a given amount of gas held at constant temperature is inversely proportional to. P1 × v1 = p2 × v2. We can write the boyle's law equation in the following way: Where p1. How Do You Calculate The Volume Of A Gas Under Pressure.
From socratic.org
A "1.32 L" volume of gas has a pressure of "1.00 atm". What will the How Do You Calculate The Volume Of A Gas Under Pressure this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature,. if you take the pressure value and multiply it by the volume value, the product is a constant (k) for a given. volume of a gas sample the sample of gas in figure 9.13 has a volume of. How Do You Calculate The Volume Of A Gas Under Pressure.
From mungfali.com
Pressure Volume And Temperature Formula How Do You Calculate The Volume Of A Gas Under Pressure you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. P1 × v1 = p2 × v2. if you take the pressure value and multiply it by the volume value, the product is a constant (k) for a given. Where p1 and v1 are initial pressure and. . How Do You Calculate The Volume Of A Gas Under Pressure.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial How Do You Calculate The Volume Of A Gas Under Pressure volume of a gas sample the sample of gas in figure 9.13 has a volume of 15.0 ml at a pressure of 13.0 psi. P1 × v1 = p2 × v2. The volume of a given amount of gas held at constant temperature is inversely proportional to. you can use the ideal gas volume calculator to find the. How Do You Calculate The Volume Of A Gas Under Pressure.
From taylorwindam1952.blogspot.com
Taylor Windam How Do You Measure Volume Of A Gas How Do You Calculate The Volume Of A Gas Under Pressure if you take the pressure value and multiply it by the volume value, the product is a constant (k) for a given. We can write the boyle's law equation in the following way: volume of a gas sample the sample of gas in figure 9.13 has a volume of 15.0 ml at a pressure of 13.0 psi. Where. How Do You Calculate The Volume Of A Gas Under Pressure.
From www.britannica.com
Ideal gas Definition, Equation, Properties, & Facts Britannica How Do You Calculate The Volume Of A Gas Under Pressure Where p1 and v1 are initial pressure and. P1 × v1 = p2 × v2. We can write the boyle's law equation in the following way: volume of a gas sample the sample of gas in figure 9.13 has a volume of 15.0 ml at a pressure of 13.0 psi. The volume of a given amount of gas held. How Do You Calculate The Volume Of A Gas Under Pressure.
From ecampusontario.pressbooks.pub
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas How Do You Calculate The Volume Of A Gas Under Pressure Where p1 and v1 are initial pressure and. if you take the pressure value and multiply it by the volume value, the product is a constant for a given amount. The volume of a given amount of gas held at constant temperature is inversely proportional to. this ideal gas law calculator will help you establish the properties of. How Do You Calculate The Volume Of A Gas Under Pressure.