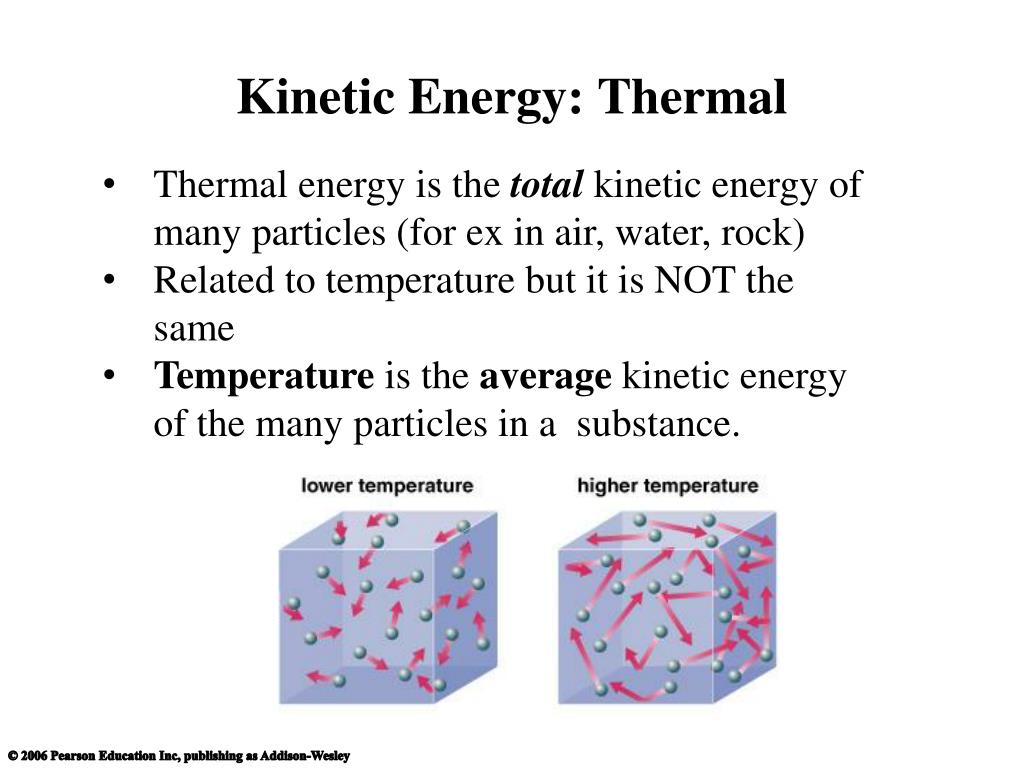
Thermal Energy Kinetic Particles . The thermal energy is the total sum of the potential energies of the particles in a system. The potential and kinetic energies that are associated with those oscillations can each be divided into three independent modes, each one. The thermal energy is the average kinetic. Thermal energy, also called heat energy or simply heat , is a type of internal energy an object is said to possess owing to the kinetic energy of its constituent particles. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object. In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random fluctuations of subatomic. Energy itself, while easy enough to define in mathematical terms, is among the more elusive quantities in physics in terms of what it fundamentally is. In a statistical mechanical account of an ideal gas, in which the molecules move independently between instantaneous collisions, the internal.
from www.slideserve.com
In a statistical mechanical account of an ideal gas, in which the molecules move independently between instantaneous collisions, the internal. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random fluctuations of subatomic. The potential and kinetic energies that are associated with those oscillations can each be divided into three independent modes, each one. Thermal energy, also called heat energy or simply heat , is a type of internal energy an object is said to possess owing to the kinetic energy of its constituent particles. The thermal energy is the total sum of the potential energies of the particles in a system. The thermal energy is the average kinetic. Energy itself, while easy enough to define in mathematical terms, is among the more elusive quantities in physics in terms of what it fundamentally is. Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object.
PPT 4.3 Conservation Laws in Astronomy PowerPoint Presentation, free
Thermal Energy Kinetic Particles The potential and kinetic energies that are associated with those oscillations can each be divided into three independent modes, each one. The potential and kinetic energies that are associated with those oscillations can each be divided into three independent modes, each one. Energy itself, while easy enough to define in mathematical terms, is among the more elusive quantities in physics in terms of what it fundamentally is. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. The thermal energy is the average kinetic. Thermal energy, also called heat energy or simply heat , is a type of internal energy an object is said to possess owing to the kinetic energy of its constituent particles. The thermal energy is the total sum of the potential energies of the particles in a system. In a statistical mechanical account of an ideal gas, in which the molecules move independently between instantaneous collisions, the internal. In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random fluctuations of subatomic. Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object.
From slideplayer.com
Heat Transfer Atmosphere Humidity Wind ppt download Thermal Energy Kinetic Particles The thermal energy is the total sum of the potential energies of the particles in a system. Energy itself, while easy enough to define in mathematical terms, is among the more elusive quantities in physics in terms of what it fundamentally is. In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in. Thermal Energy Kinetic Particles.
From sciencing.com
Thermal Energy Definition, Equation, Types (w/ Diagram & Examples Thermal Energy Kinetic Particles The thermal energy is the average kinetic. Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object. In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random fluctuations of subatomic. The kinetic temperature is the variable needed for subjects like heat transfer,. Thermal Energy Kinetic Particles.
From www.sciencefacts.net
Thermal (Heat) Energy Definition, Examples, Equations, and Units Thermal Energy Kinetic Particles The thermal energy is the total sum of the potential energies of the particles in a system. In a statistical mechanical account of an ideal gas, in which the molecules move independently between instantaneous collisions, the internal. Energy itself, while easy enough to define in mathematical terms, is among the more elusive quantities in physics in terms of what it. Thermal Energy Kinetic Particles.
From www.pinterest.com
Thermal Energy Easy Science Thermal energy, Chemistry basics, Learn Thermal Energy Kinetic Particles In a statistical mechanical account of an ideal gas, in which the molecules move independently between instantaneous collisions, the internal. Energy itself, while easy enough to define in mathematical terms, is among the more elusive quantities in physics in terms of what it fundamentally is. In the particle model of thermal energy we describe thermal energy of a macroscopic solid. Thermal Energy Kinetic Particles.
From slideplayer.com
Forces, Motion, and Energy ppt download Thermal Energy Kinetic Particles The thermal energy is the total sum of the potential energies of the particles in a system. The potential and kinetic energies that are associated with those oscillations can each be divided into three independent modes, each one. In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random fluctuations. Thermal Energy Kinetic Particles.
From www.pinterest.com
How thermals form. Science diagram showing how molecules react during Thermal Energy Kinetic Particles In a statistical mechanical account of an ideal gas, in which the molecules move independently between instantaneous collisions, the internal. The thermal energy is the average kinetic. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. In the particle model of thermal energy we describe thermal energy. Thermal Energy Kinetic Particles.
From slideplayer.com
Thermal Energy and Heat ppt download Thermal Energy Kinetic Particles In a statistical mechanical account of an ideal gas, in which the molecules move independently between instantaneous collisions, the internal. Thermal energy, also called heat energy or simply heat , is a type of internal energy an object is said to possess owing to the kinetic energy of its constituent particles. Energy itself, while easy enough to define in mathematical. Thermal Energy Kinetic Particles.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Thermal Energy Kinetic Particles In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random fluctuations of subatomic. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Thermal energy, also called heat energy or simply heat , is a type of. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT Temperature measures the average energy of PowerPoint Thermal Energy Kinetic Particles The thermal energy is the total sum of the potential energies of the particles in a system. Thermal energy, also called heat energy or simply heat , is a type of internal energy an object is said to possess owing to the kinetic energy of its constituent particles. The kinetic temperature is the variable needed for subjects like heat transfer,. Thermal Energy Kinetic Particles.
From www.dreamstime.com
Thermal Energy Physics Definition, Example with Water and Thermal Energy Kinetic Particles The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. In a statistical mechanical account of an ideal gas, in which the molecules move independently between instantaneous collisions, the internal. The thermal energy is the average kinetic. Energy itself, while easy enough to define in mathematical terms, is. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT Energy and Matter PowerPoint Presentation, free download ID6834725 Thermal Energy Kinetic Particles The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object. In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT The Nature of Matter PowerPoint Presentation, free download ID Thermal Energy Kinetic Particles Thermal energy, also called heat energy or simply heat , is a type of internal energy an object is said to possess owing to the kinetic energy of its constituent particles. The potential and kinetic energies that are associated with those oscillations can each be divided into three independent modes, each one. The thermal energy is the average kinetic. In. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT Physical Science Chapter 6 PowerPoint Presentation, free download Thermal Energy Kinetic Particles The potential and kinetic energies that are associated with those oscillations can each be divided into three independent modes, each one. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Thermal energy, also called heat energy or simply heat , is a type of internal energy an. Thermal Energy Kinetic Particles.
From www.youtube.com
6.1 Temperature and energy (SL) YouTube Thermal Energy Kinetic Particles The thermal energy is the average kinetic. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Thermal energy, also called heat energy or simply heat , is a type of internal energy an object is said to possess owing to the kinetic energy of its constituent particles.. Thermal Energy Kinetic Particles.
From ar.inspiredpencil.com
Thermal Energy Of Particles Diagram Thermal Energy Kinetic Particles Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object. The thermal energy is the average kinetic. Energy itself, while easy enough to define in mathematical terms, is among the more elusive quantities in physics in terms of what it fundamentally is. The kinetic temperature is the variable needed for subjects like heat. Thermal Energy Kinetic Particles.
From sciencenotes.org
What Is Energy? Energy Examples Thermal Energy Kinetic Particles In a statistical mechanical account of an ideal gas, in which the molecules move independently between instantaneous collisions, the internal. In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random fluctuations of subatomic. The potential and kinetic energies that are associated with those oscillations can each be divided into. Thermal Energy Kinetic Particles.
From www.youtube.com
Energy, Temperature and Particle Speeds YouTube Thermal Energy Kinetic Particles In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random fluctuations of subatomic. The thermal energy is the total sum of the potential energies of the particles in a system. Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object. Thermal energy,. Thermal Energy Kinetic Particles.
From studylib.net
Thermal Equilibrium energy of a particle KE = Temperature Thermal Energy Kinetic Particles The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. The thermal energy is the total sum of the potential energies of the particles in a system. The thermal energy is the average kinetic. Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT Theory of Matter PowerPoint Presentation, free download Thermal Energy Kinetic Particles Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object. In a statistical mechanical account of an ideal gas, in which the molecules move independently between instantaneous collisions, the internal. Energy itself, while easy enough to define in mathematical terms, is among the more elusive quantities in physics in terms of what it. Thermal Energy Kinetic Particles.
From ar.inspiredpencil.com
Energy Temperature Thermal Energy Kinetic Particles The thermal energy is the total sum of the potential energies of the particles in a system. Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object. Energy itself, while easy enough to define in mathematical terms, is among the more elusive quantities in physics in terms of what it fundamentally is. The. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT What is the difference between heat and temperature? PowerPoint Thermal Energy Kinetic Particles Energy itself, while easy enough to define in mathematical terms, is among the more elusive quantities in physics in terms of what it fundamentally is. The potential and kinetic energies that are associated with those oscillations can each be divided into three independent modes, each one. The kinetic temperature is the variable needed for subjects like heat transfer, because it. Thermal Energy Kinetic Particles.
From energyeducation.ca
Thermal conduction Energy Education Thermal Energy Kinetic Particles The thermal energy is the average kinetic. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object. Thermal energy, also called heat energy or simply heat , is a type of. Thermal Energy Kinetic Particles.
From slideplayer.com
Energy. ppt download Thermal Energy Kinetic Particles The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object. The thermal energy is the total sum of the potential energies of the particles in a system. In a statistical mechanical. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT A measure of the average energy of the particles in a Thermal Energy Kinetic Particles The potential and kinetic energies that are associated with those oscillations can each be divided into three independent modes, each one. Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object. The thermal energy is the average kinetic. Energy itself, while easy enough to define in mathematical terms, is among the more elusive. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT Matter Theory Solid Liquid Gas Plasma PowerPoint Thermal Energy Kinetic Particles In a statistical mechanical account of an ideal gas, in which the molecules move independently between instantaneous collisions, the internal. The thermal energy is the average kinetic. Energy itself, while easy enough to define in mathematical terms, is among the more elusive quantities in physics in terms of what it fundamentally is. In the particle model of thermal energy we. Thermal Energy Kinetic Particles.
From slideplayer.com
Total energy of particles ppt download Thermal Energy Kinetic Particles The thermal energy is the total sum of the potential energies of the particles in a system. In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random fluctuations of subatomic. The thermal energy is the average kinetic. Thermal energy consists of the microscopic kinetic and potential energies of the. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT Solids, Liquids, and Gases PowerPoint Presentation, free download Thermal Energy Kinetic Particles The thermal energy is the total sum of the potential energies of the particles in a system. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID5445725 Thermal Energy Kinetic Particles In a statistical mechanical account of an ideal gas, in which the molecules move independently between instantaneous collisions, the internal. Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object. The thermal energy is the average kinetic. The potential and kinetic energies that are associated with those oscillations can each be divided into. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download Thermal Energy Kinetic Particles Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object. In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random fluctuations of subatomic. The thermal energy is the total sum of the potential energies of the particles in a system. Energy itself,. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT 4.3 Conservation Laws in Astronomy PowerPoint Presentation, free Thermal Energy Kinetic Particles The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. The potential and kinetic energies that are associated with those oscillations can each be divided into three independent modes, each one. Thermal energy consists of the microscopic kinetic and potential energies of the microscopic particles comprising an object.. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT Thermal Energy & Matter PowerPoint Presentation, free download Thermal Energy Kinetic Particles The thermal energy is the total sum of the potential energies of the particles in a system. The potential and kinetic energies that are associated with those oscillations can each be divided into three independent modes, each one. In a statistical mechanical account of an ideal gas, in which the molecules move independently between instantaneous collisions, the internal. The thermal. Thermal Energy Kinetic Particles.
From mmerevise.co.uk
Thermal Energy Transfer Questions and Revision MME Thermal Energy Kinetic Particles In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random fluctuations of subatomic. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. The thermal energy is the total sum of the potential energies of the particles. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT Thermal Energy A. Temperature & Heat 1. Temperature is related to Thermal Energy Kinetic Particles Energy itself, while easy enough to define in mathematical terms, is among the more elusive quantities in physics in terms of what it fundamentally is. Thermal energy, also called heat energy or simply heat , is a type of internal energy an object is said to possess owing to the kinetic energy of its constituent particles. The potential and kinetic. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT Unit 6 PowerPoint Presentation, free download ID1516058 Thermal Energy Kinetic Particles The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. In the particle model of thermal energy we describe thermal energy of a macroscopic solid of liquid in terms of random fluctuations of subatomic. Energy itself, while easy enough to define in mathematical terms, is among the more. Thermal Energy Kinetic Particles.
From www.slideserve.com
PPT How to Use This Presentation PowerPoint Presentation, free Thermal Energy Kinetic Particles The potential and kinetic energies that are associated with those oscillations can each be divided into three independent modes, each one. Thermal energy, also called heat energy or simply heat , is a type of internal energy an object is said to possess owing to the kinetic energy of its constituent particles. The thermal energy is the average kinetic. In. Thermal Energy Kinetic Particles.