Copper Ii Hydroxide Balanced Equation . Balance a chemical equation when given the unbalanced equation. Explain the roles of subscripts and coefficients in chemical equations. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The balanced equation will be calculated along with the. Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Let's balance this equation using the inspection method. Enter an equation of an ionic chemical equation and press the balance button. Explain the role of the law of conservation of mass in a chemical reaction. For each element, we check if the number of atoms is balanced on both sides of the. Chemical equation (h2o = h + o) 🛠️
from www.chegg.com
Balance a chemical equation when given the unbalanced equation. The balanced equation will be calculated along with the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Chemical equation (h2o = h + o) 🛠️ Explain the roles of subscripts and coefficients in chemical equations. For each element, we check if the number of atoms is balanced on both sides of the. Let's balance this equation using the inspection method. Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Enter an equation of an ionic chemical equation and press the balance button. Explain the role of the law of conservation of mass in a chemical reaction.
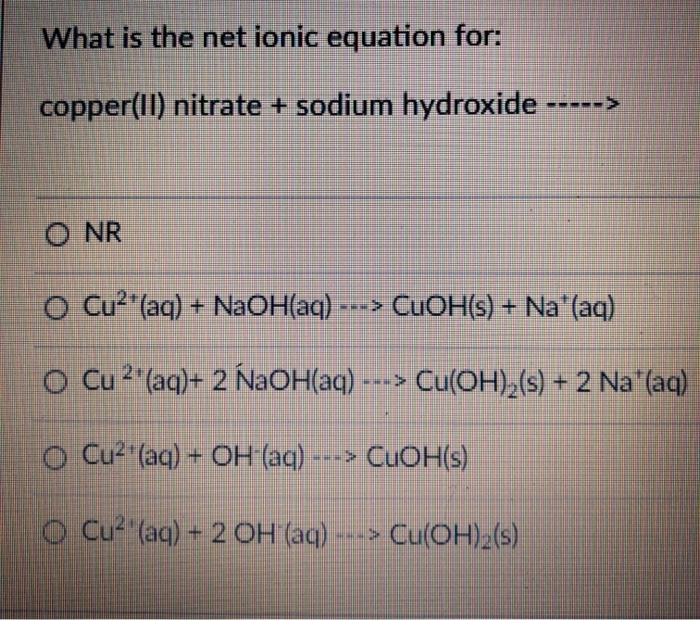
Solved What is the net ionic equation for copper(II)
Copper Ii Hydroxide Balanced Equation Let's balance this equation using the inspection method. Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Chemical equation (h2o = h + o) 🛠️ Balance a chemical equation when given the unbalanced equation. Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Enter an equation of an ionic chemical equation and press the balance button. For each element, we check if the number of atoms is balanced on both sides of the. The balanced equation will be calculated along with the. Let's balance this equation using the inspection method. Explain the role of the law of conservation of mass in a chemical reaction.
From www.numerade.com
SOLVEDExample Write Out the balanced chemical equation for the reaction of copper metal with Copper Ii Hydroxide Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. Chemical equation (h2o = h + o) 🛠️ For each element, we check if the number of atoms is balanced on both sides of the. Explain the role. Copper Ii Hydroxide Balanced Equation.
From www.bartleby.com
Answered Consider the insoluble compound… bartleby Copper Ii Hydroxide Balanced Equation Explain the role of the law of conservation of mass in a chemical reaction. Let's balance this equation using the inspection method. Chemical equation (h2o = h + o) 🛠️ Enter an equation of an ionic chemical equation and press the balance button. Balance a chemical equation when given the unbalanced equation. The balanced equation will be calculated along with. Copper Ii Hydroxide Balanced Equation.
From www.chegg.com
Solved Consider the insoluble compound copper(II) hydroxide Copper Ii Hydroxide Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Enter an equation of an ionic chemical equation and press the balance button. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Chemical equation (h2o =. Copper Ii Hydroxide Balanced Equation.
From www.youtube.com
CuCl2+NaOH=Cu(OH)2+NaClCopper (ii)chloride+Sodium hydroxide=Copper (ii) hydroxide Balanced Copper Ii Hydroxide Balanced Equation Balance a chemical equation when given the unbalanced equation. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Explain the role of the law of conservation of mass in a chemical reaction. Enter an equation of an ionic chemical equation and press the balance button. The chemical equation described in section 4.1 is balanced,. Copper Ii Hydroxide Balanced Equation.
From bnrc.springeropen.com
Copper(II) oxide nanocatalyst preparation and characterization green chemistry route Bulletin Copper Ii Hydroxide Balanced Equation Let's balance this equation using the inspection method. Explain the role of the law of conservation of mass in a chemical reaction. The balanced equation will be calculated along with the. Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved. Copper Ii Hydroxide Balanced Equation.
From www.t3db.ca
T3DB Copper(II) hydroxide Copper Ii Hydroxide Balanced Equation Let's balance this equation using the inspection method. Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Learn how to balance chemical equations using the law of conservation of mass and the method of trial and. Copper Ii Hydroxide Balanced Equation.
From www.chegg.com
Solved 3. Copper (II) hydroxide is amphoteric. What would Copper Ii Hydroxide Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Explain the role of the law of conservation of mass in a chemical reaction. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). For each element, we check if the number of atoms is balanced on both sides of the. Balance a chemical equation. Copper Ii Hydroxide Balanced Equation.
From www.youtube.com
Lab Write Balanced Molecular, Total Ionic Equation, and Net Ionic Equation of Copper(II Copper Ii Hydroxide Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Explain the role of the law of conservation of mass in a chemical reaction. Let's balance this equation using the inspection method. Balance a chemical equation when given the unbalanced equation. Explain the roles of subscripts and coefficients in chemical. Copper Ii Hydroxide Balanced Equation.
From www.numerade.com
SOLVED write Balanced equation for copper metal and oxygen gas and acetic acid react to form Copper Ii Hydroxide Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). For each element, we check if the number of atoms is balanced on both sides of the. Balance a chemical equation when given the unbalanced equation. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved. Copper Ii Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Q2Write balanced equations for the following reactions (start with the skeleton equation Copper Ii Hydroxide Balanced Equation The balanced equation will be calculated along with the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Balance a chemical equation when given the unbalanced equation. Explain the role of the law of conservation of mass in a chemical reaction. Explain the roles of subscripts and coefficients in chemical equations. Let's balance this. Copper Ii Hydroxide Balanced Equation.
From www.slideserve.com
PPT Polyatomic Ions and Their Compounds PowerPoint Presentation ID153488 Copper Ii Hydroxide Balanced Equation The balanced equation will be calculated along with the. Explain the role of the law of conservation of mass in a chemical reaction. For each element, we check if the number of atoms is balanced on both sides of the. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in. Copper Ii Hydroxide Balanced Equation.
From www.youtube.com
How to Write the Equation for Cu(OH)2 + H2O YouTube Copper Ii Hydroxide Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Let's balance this equation using the inspection method. For each element, we check if the number of atoms is balanced on both sides of the. Enter an equation of an ionic chemical equation and press the balance button. Explain the role of the law of conservation of mass in a. Copper Ii Hydroxide Balanced Equation.
From www.youtube.com
How to Write the Formula for Copper (II) hydroxide YouTube Copper Ii Hydroxide Balanced Equation The balanced equation will be calculated along with the. Let's balance this equation using the inspection method. Explain the role of the law of conservation of mass in a chemical reaction. Balance a chemical equation when given the unbalanced equation. For each element, we check if the number of atoms is balanced on both sides of the. Enter an equation. Copper Ii Hydroxide Balanced Equation.
From www.youtube.com
of CopperII Carbonate Hydroxide Hydrate Lab Video YouTube Copper Ii Hydroxide Balanced Equation The balanced equation will be calculated along with the. Let's balance this equation using the inspection method. Chemical equation (h2o = h + o) 🛠️ Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Use the calculator below to balance chemical equations and determine the type of reaction (instructions).. Copper Ii Hydroxide Balanced Equation.
From www.numerade.com
SOLVED REAcTIoN 124 COPPER SULFATE (Cuso,) AND AMMONIUM HYDROXIDE C (Write balanced equation Copper Ii Hydroxide Balanced Equation Balance a chemical equation when given the unbalanced equation. Enter an equation of an ionic chemical equation and press the balance button. Let's balance this equation using the inspection method. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Explain the roles of subscripts and coefficients in chemical equations. For each element, we check. Copper Ii Hydroxide Balanced Equation.
From www.youtube.com
How to Balance CuO + H2 = Cu + H2O Copper (II) Oxide and Hydrogen Gas YouTube Copper Ii Hydroxide Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Let's balance. Copper Ii Hydroxide Balanced Equation.
From www.toppr.com
Write balanced chemical equations for the following word equationCopper + Oxygen → Copper (II Copper Ii Hydroxide Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Balance a chemical equation when given the unbalanced equation. Explain the role of the law of conservation of mass in a chemical reaction. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each. Copper Ii Hydroxide Balanced Equation.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free download ID2858272 Copper Ii Hydroxide Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Let's balance this equation using the inspection method. Chemical equation (h2o = h + o) 🛠️ The balanced equation will be calculated along with the. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Enter an. Copper Ii Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Write the balanced molecular chemical equation for the reaction in aqueous solution for Copper Ii Hydroxide Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. For each element, we check if the number of atoms is balanced on both sides of the. The balanced equation will be calculated along. Copper Ii Hydroxide Balanced Equation.
From www.chegg.com
Solved 3. Copper (II) hydroxide is amphoteric. What would Copper Ii Hydroxide Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. Explain the roles of subscripts and coefficients in chemical equations. Explain the role of the law of conservation of mass in a chemical reaction. Balance a chemical equation when given the unbalanced equation. The balanced equation will be calculated along with the. Chemical equation (h2o = h. Copper Ii Hydroxide Balanced Equation.
From www.youtube.com
How to Balance Copper (II) sulfate + Ammonium hydroxide YouTube Copper Ii Hydroxide Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The balanced equation will be calculated along with the. Let's balance this equation using the inspection method. Enter an equation of an ionic chemical equation and press the balance button. Chemical equation (h2o = h + o). Copper Ii Hydroxide Balanced Equation.
From www.youtube.com
Type of Reaction for Cu(OH)2 = CuO + H2O YouTube Copper Ii Hydroxide Balanced Equation Balance a chemical equation when given the unbalanced equation. Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The balanced equation will be calculated along with. Copper Ii Hydroxide Balanced Equation.
From www.youtube.com
How to find the percent composition of Cu(OH)2 (Copper (II) Hydroxide) YouTube Copper Ii Hydroxide Balanced Equation Balance a chemical equation when given the unbalanced equation. Enter an equation of an ionic chemical equation and press the balance button. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Use the calculator below to balance chemical equations and determine the type of reaction (instructions).. Copper Ii Hydroxide Balanced Equation.
From www.chegg.com
Solved 1. Write the balanced chemical equation for the Copper Ii Hydroxide Balanced Equation Let's balance this equation using the inspection method. Enter an equation of an ionic chemical equation and press the balance button. Explain the role of the law of conservation of mass in a chemical reaction. For each element, we check if the number of atoms is balanced on both sides of the. The balanced equation will be calculated along with. Copper Ii Hydroxide Balanced Equation.
From www.youtube.com
Copper in Copper I hydroxide and Copper II hydroxide YouTube Copper Ii Hydroxide Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Balance a chemical equation when given the unbalanced equation. Let's balance this equation using the inspection method. Chemical equation (h2o = h + o) 🛠️ For each element, we check if the number of atoms is balanced on both sides of the. Enter an equation. Copper Ii Hydroxide Balanced Equation.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Observations... Course Hero Copper Ii Hydroxide Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Enter an equation of an ionic chemical equation and press the balance button. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The balanced equation will be calculated along with the. Balance a chemical equation when. Copper Ii Hydroxide Balanced Equation.
From www.chegg.com
Solved Consider the insoluble compound copper(II) hydroxide, Copper Ii Hydroxide Balanced Equation For each element, we check if the number of atoms is balanced on both sides of the. Let's balance this equation using the inspection method. Chemical equation (h2o = h + o) 🛠️ Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. The balanced equation will be calculated along. Copper Ii Hydroxide Balanced Equation.
From www.youtube.com
of Copper(II) Carbonate Hydroxide Hydrate in LateNiteLabs YouTube Copper Ii Hydroxide Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. For each element, we check if the number of atoms is balanced on both sides of the. Enter an equation of an ionic chemical equation and press the balance button. Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error.. Copper Ii Hydroxide Balanced Equation.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper Ii Hydroxide Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Explain the roles of subscripts and coefficients in chemical equations. Explain the role of the law of conservation of mass in a chemical reaction. The balanced equation will be calculated along with the. Let's balance this equation. Copper Ii Hydroxide Balanced Equation.
From www.toppr.com
Write balanced chemical equations the following word equationCopper +Oxygen → Copper (II) oxide Copper Ii Hydroxide Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Enter an. Copper Ii Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Balance the equations or write the balanced chemical for the following (2 Marks) A Copper Ii Hydroxide Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Balance a chemical equation when given the unbalanced equation. Let's balance this equation using the inspection method. Explain the role of the law of conservation of mass in a chemical reaction. The chemical equation described in section 4.1 is balanced,. Copper Ii Hydroxide Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Cu(OH)2 = CuO + H2O YouTube Copper Ii Hydroxide Balanced Equation For each element, we check if the number of atoms is balanced on both sides of the. Explain the roles of subscripts and coefficients in chemical equations. Chemical equation (h2o = h + o) 🛠️ The balanced equation will be calculated along with the. Balance a chemical equation when given the unbalanced equation. Let's balance this equation using the inspection. Copper Ii Hydroxide Balanced Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation. Copper + nitric acid to cop Copper Ii Hydroxide Balanced Equation Balance a chemical equation when given the unbalanced equation. Let's balance this equation using the inspection method. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Explain the roles of subscripts. Copper Ii Hydroxide Balanced Equation.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Copper Ii Hydroxide Balanced Equation For each element, we check if the number of atoms is balanced on both sides of the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). The balanced equation will be calculated along with the. Explain the roles of subscripts and coefficients in chemical equations. Enter an equation of an ionic chemical equation and. Copper Ii Hydroxide Balanced Equation.
From www.numerade.com
SOLVED REACTION5COPPER SULFATE(CuSO,)AND SODIUM HYDROXIDE(NaOH) (Write balanced equation and Copper Ii Hydroxide Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Chemical equation (h2o = h + o) 🛠️ The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each. Copper Ii Hydroxide Balanced Equation.