Titration Of Naoh . Solve stoichiometry problems in the solution. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The titration process can be observed in the video below. Hydrochloric acid reacts with sodium hydroxide. Hcl + naoh → nacl + h 2 o. First determine the moles of \(\ce{naoh}\) in the reaction. Describe what is occurring on a molecular level during the titration of a weak acid, such as acetic acid, with a strong base, such as naoh, at the following points along the. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titrate with naoh solution till the first color change. According to the reaction equation. Includes kit list and safety instructions.
from www.chegg.com
Hcl + naoh → nacl + h 2 o. Solve stoichiometry problems in the solution. Describe what is occurring on a molecular level during the titration of a weak acid, such as acetic acid, with a strong base, such as naoh, at the following points along the. The titration process can be observed in the video below. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. According to the reaction equation. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Hydrochloric acid reacts with sodium hydroxide. Titrate with naoh solution till the first color change. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid.
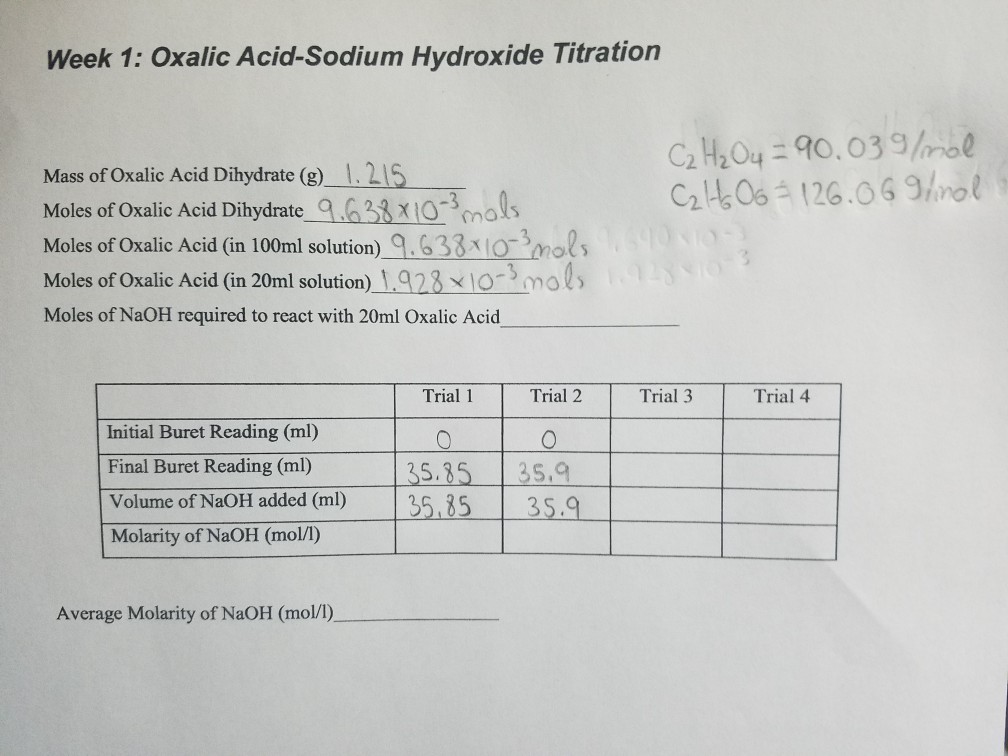
Solved Week 1 Oxalic AcidSodium Hydroxide Titration Mass
Titration Of Naoh From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titrate with naoh solution till the first color change. The titration process can be observed in the video below. Solve stoichiometry problems in the solution. Hcl + naoh → nacl + h 2 o. Hydrochloric acid reacts with sodium hydroxide. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. First determine the moles of \(\ce{naoh}\) in the reaction. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Describe what is occurring on a molecular level during the titration of a weak acid, such as acetic acid, with a strong base, such as naoh, at the following points along the. Includes kit list and safety instructions. According to the reaction equation.
From www.sliderbase.com
Titration Titration Of Naoh From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Hydrochloric acid reacts with sodium hydroxide. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. The titration process can be. Titration Of Naoh.
From askfilo.com
Consider titration of NaOH solution versus 1.25M oxalic acid solution. At.. Titration Of Naoh First determine the moles of \(\ce{naoh}\) in the reaction. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. Solve stoichiometry problems in the solution.. Titration Of Naoh.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Titration Of Naoh Solve stoichiometry problems in the solution. According to the reaction equation. Hydrochloric acid reacts with sodium hydroxide. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. First determine the moles of \(\ce{naoh}\) in the reaction. Titrate with naoh solution till the first color change. A measured volume of the solution to. Titration Of Naoh.
From mungfali.com
HCl NaOH Titration Titration Of Naoh Solve stoichiometry problems in the solution. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. Describe what is occurring on a molecular level during the titration of a weak acid, such as acetic acid, with a strong base, such as naoh, at the following points along the. The. Titration Of Naoh.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Of Naoh Hydrochloric acid reacts with sodium hydroxide. Hcl + naoh → nacl + h 2 o. Titrate with naoh solution till the first color change. According to the reaction equation. Describe what is occurring on a molecular level during the titration of a weak acid, such as acetic acid, with a strong base, such as naoh, at the following points along. Titration Of Naoh.
From www.chegg.com
Solved Use the following titration curve for the first four Titration Of Naoh Hcl + naoh → nacl + h 2 o. The titration process can be observed in the video below. According to the reaction equation. First determine the moles of \(\ce{naoh}\) in the reaction. Titrate with naoh solution till the first color change. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of. Titration Of Naoh.
From lessonlibraryemersed.z13.web.core.windows.net
Titration Practical Questions And Answers Titration Of Naoh A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. Hcl + naoh → nacl + h 2 o. First determine the moles of \(\ce{naoh}\) in the reaction. Solve stoichiometry problems in the solution. Describe what is occurring on a molecular level during the titration of a weak acid,. Titration Of Naoh.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Of Naoh Includes kit list and safety instructions. Hcl + naoh → nacl + h 2 o. Titrate with naoh solution till the first color change. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Describe what is occurring on a molecular level during the titration of a weak acid, such. Titration Of Naoh.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Of Naoh Solve stoichiometry problems in the solution. Hcl + naoh → nacl + h 2 o. Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titrate with naoh solution till the first color change. A measured volume of the solution to be titrated, in this case,. Titration Of Naoh.
From www.chegg.com
Solved Week 1 Oxalic AcidSodium Hydroxide Titration Mass Titration Of Naoh Hcl + naoh → nacl + h 2 o. First determine the moles of \(\ce{naoh}\) in the reaction. According to the reaction equation. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and. Titration Of Naoh.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Titration Of Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Describe what is occurring on a molecular level during the titration of a weak acid, such as acetic acid, with a strong base, such as naoh, at the following points along the. The titration process can be observed in the video below.. Titration Of Naoh.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Titration Of Naoh A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. Includes kit list and safety instructions. The titration process can be observed in the video below. Titrate with naoh solution till the first color change. Use this class practical to explore titration, producing the salt sodium chloride with sodium. Titration Of Naoh.
From socratic.org
What is the Ka value of this citric acid + NaOH titration? Socratic Titration Of Naoh Includes kit list and safety instructions. According to the reaction equation. Hcl + naoh → nacl + h 2 o. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Hydrochloric acid reacts with sodium hydroxide. First determine the moles of. Titration Of Naoh.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titration Of Naoh A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. Hcl + naoh → nacl + h 2 o. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. According. Titration Of Naoh.
From www.chegg.com
TitrationStandardization of Sodium Hydroxide Titration Of Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Solve stoichiometry problems in the solution. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The titration process can be observed in the video below. Includes kit list and. Titration Of Naoh.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Of Naoh The titration process can be observed in the video below. Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide. Titrate with naoh solution till the first color change. First determine the moles of \(\ce{naoh}\) in the reaction. Describe what. Titration Of Naoh.
From mungfali.com
HCl NaOH Titration Titration Of Naoh Hydrochloric acid reacts with sodium hydroxide. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. Hcl + naoh → nacl + h 2 o. According to the reaction equation. First determine the moles of \(\ce{naoh}\) in the reaction. The titration process can be observed in the video below.. Titration Of Naoh.
From www.numerade.com
Titration for Acetic Acid in Vinegar Lab Report Exercise 1 Titration Of Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Hydrochloric acid reacts with sodium hydroxide. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. Titration Of Naoh.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Of Naoh Solve stoichiometry problems in the solution. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide. Includes kit list and safety instructions. Hcl + naoh → nacl + h 2 o. A titration is carried. Titration Of Naoh.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Titration Of Naoh Includes kit list and safety instructions. Solve stoichiometry problems in the solution. Hydrochloric acid reacts with sodium hydroxide. According to the reaction equation. Describe what is occurring on a molecular level during the titration of a weak acid, such as acetic acid, with a strong base, such as naoh, at the following points along the. A measured volume of the. Titration Of Naoh.
From plotly.com
Titration HNO3 with NaOH scatter chart made by Zhaoay plotly Titration Of Naoh Describe what is occurring on a molecular level during the titration of a weak acid, such as acetic acid, with a strong base, such as naoh, at the following points along the. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. According to the reaction equation. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is. Titration Of Naoh.
From www.youtube.com
Titration Acetic Acid with NaOH YouTube Titration Of Naoh Describe what is occurring on a molecular level during the titration of a weak acid, such as acetic acid, with a strong base, such as naoh, at the following points along the. First determine the moles of \(\ce{naoh}\) in the reaction. The titration process can be observed in the video below. A titration is carried out for 25.00 ml of. Titration Of Naoh.
From www.studocu.com
Titration of HCl with Na OH C12510 Titration of HCl with NaOH C125 Titration Of Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. First determine the moles of \(\ce{naoh}\) in the reaction. Includes kit list and safety instructions. The titration process can be observed. Titration Of Naoh.
From askfilo.com
Consider titration of NaOH solution versus 1.25 M oxalic acid solution. A.. Titration Of Naoh Includes kit list and safety instructions. The titration process can be observed in the video below. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. Titrate with naoh solution till the first color change. Use this class practical to explore titration, producing the salt sodium chloride with sodium. Titration Of Naoh.
From www.chegg.com
Titration of sulfuric acid Average Volume of NaOH = Titration Of Naoh Solve stoichiometry problems in the solution. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Hydrochloric acid reacts with sodium hydroxide. The titration process can be observed in the video below. A measured volume of the solution to be titrated,. Titration Of Naoh.
From www.youtube.com
Titration Of NaOH with Oxalic Acid ( Class XI, Practical1) YouTube Titration Of Naoh Hydrochloric acid reacts with sodium hydroxide. Solve stoichiometry problems in the solution. Hcl + naoh → nacl + h 2 o. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Includes kit list and safety instructions. First determine the moles. Titration Of Naoh.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Of Naoh Hcl + naoh → nacl + h 2 o. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. According to the reaction equation. A titration is carried out for 25.00 ml. Titration Of Naoh.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Of Naoh From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. Hydrochloric acid reacts with sodium hydroxide. First determine the moles of \(\ce{naoh}\) in the reaction. Titrate with naoh solution till the first color change. The titration process can. Titration Of Naoh.
From www.slideserve.com
PPT Acidbase titration PowerPoint Presentation, free download ID Titration Of Naoh The titration process can be observed in the video below. Hcl + naoh → nacl + h 2 o. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titrate with naoh solution till the first color change. Includes kit list and safety instructions. According to the reaction equation. From the mole. Titration Of Naoh.
From mungfali.com
HCl NaOH Titration Titration Of Naoh Hcl + naoh → nacl + h 2 o. Titrate with naoh solution till the first color change. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. First determine. Titration Of Naoh.
From mavink.com
Acetic Acid And Naoh Titration Titration Of Naoh The titration process can be observed in the video below. Solve stoichiometry problems in the solution. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. From the mole ratio, calculate the. Titration Of Naoh.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Titration Of Naoh The titration process can be observed in the video below. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Hcl + naoh → nacl + h 2 o. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. First. Titration Of Naoh.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Of Naoh Hcl + naoh → nacl + h 2 o. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide. According to the reaction equation. Solve. Titration Of Naoh.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Of Naoh Titrate with naoh solution till the first color change. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Includes kit list and safety instructions. First determine the moles of \(\ce{naoh}\) in the reaction. Hydrochloric acid reacts with sodium hydroxide. The titration process can be observed in the video below. A measured volume of the solution to be titrated,. Titration Of Naoh.
From www.tutormyself.com
(f) Acids, alkalis and titrations Archives TutorMyself Chemistry Titration Of Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. According to the reaction equation. A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, ch 3 cooh (aq) is. Titrate with naoh solution till. Titration Of Naoh.