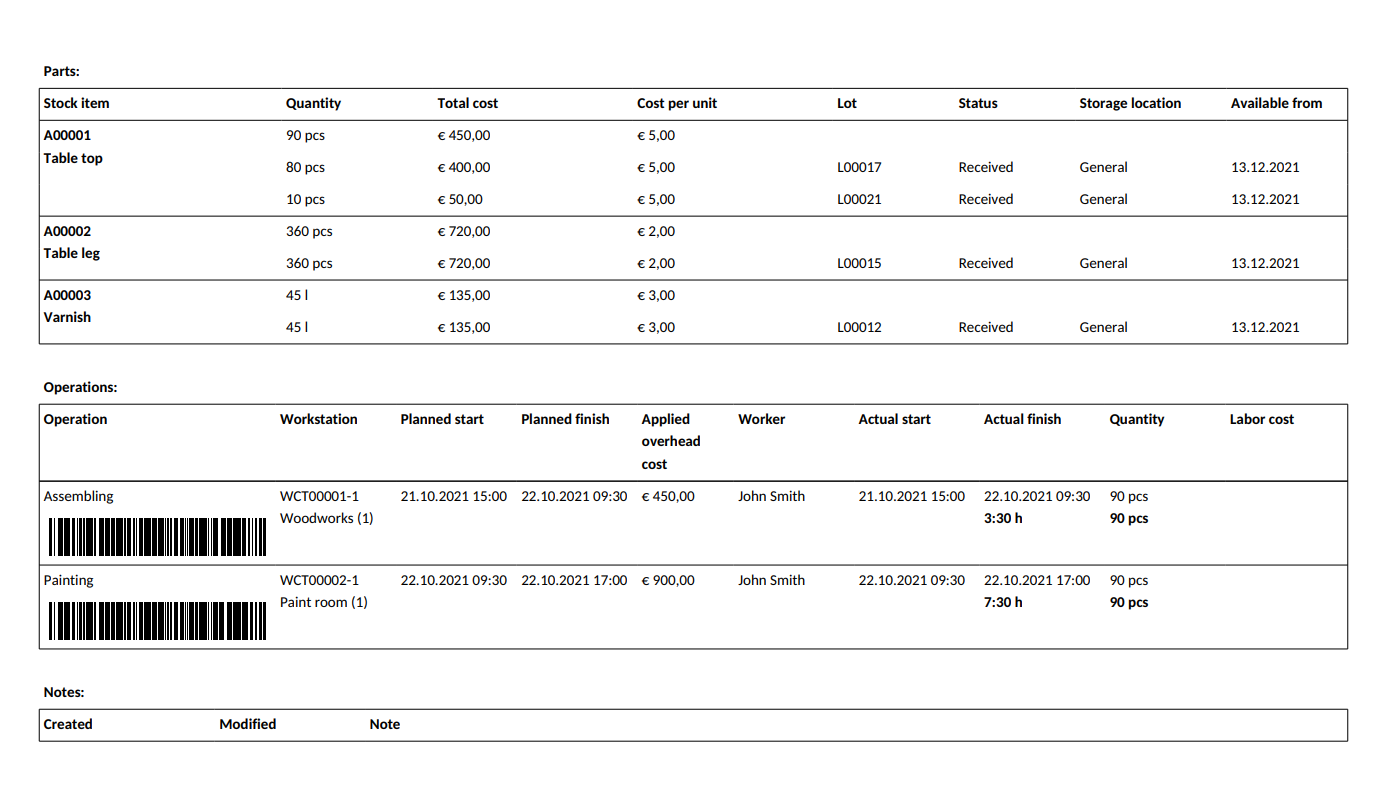
Master Batch Record Definition Fda . (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. 225.102 master record file and production records. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. the record shall include: (a) the master record file provides the complete. 211.186 master production and control records. (1) the name of the type a medicated article (s) and a specimen copy of its label. (a) to assure uniformity from batch to batch, master. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the.
from manufacturing-software-blog.mrpeasy.com
a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. the record shall include: (1) the name of the type a medicated article (s) and a specimen copy of its label. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) the master record file provides the complete. 211.186 master production and control records. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and. 225.102 master record file and production records.
How to Keep Perfect Batch Records? MRPeasy
Master Batch Record Definition Fda the batch production record should be checked before issuance to ensure that it is the correct version and a legible. the record shall include: (a) to assure uniformity from batch to batch, master. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and. 211.186 master production and control records. (a) the master record file provides the complete. for drug products, a master batch record covering the entire process including original inoculum, propagation,. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. 225.102 master record file and production records. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. (1) the name of the type a medicated article (s) and a specimen copy of its label.
From sgsystemsglobal.com
Electronic Batch Record (EBR) Batch Manufacturing Record (MBR) Master Batch Record Definition Fda the batch production record should be checked before issuance to ensure that it is the correct version and a legible. (a) to assure uniformity from batch to batch, master. for drug products, a master batch record covering the entire process including original inoculum, propagation,. the record shall include: (1) the name of the type a medicated article. Master Batch Record Definition Fda.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Master Batch Record Definition Fda 225.102 master record file and production records. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. Master Batch Record Definition Fda.
From www.youtube.com
Batch Formula Record and Master Formula Record YouTube Master Batch Record Definition Fda (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. 225.102 master record file and production records. (a) to assure uniformity from batch to batch, master. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) an accurate reproduction of the appropriate master production or control. Master Batch Record Definition Fda.
From datamyte.com
Master Batch Record Your Essential Guide DataMyte Master Batch Record Definition Fda the batch production record should be checked before issuance to ensure that it is the correct version and a legible. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (1) the name of the type a medicated article (s) and a specimen copy of its label. the record shall include: 225.102. Master Batch Record Definition Fda.
From dxohfldma.blob.core.windows.net
Master Batch Record Traduction at Ronnie Jackson blog Master Batch Record Definition Fda (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and. (a) the master record file provides the complete. (a) to assure uniformity from batch to batch, master. 211.186 master production and control records. (1) the name. Master Batch Record Definition Fda.
From farmasiindustri.com
Perbedaan Batch Record vs electronic Batch Record di Industri Farmasi Master Batch Record Definition Fda 211.186 master production and control records. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. (1) the name of the type a medicated article (s) and a specimen copy of its label. for drug products, a master batch record covering the entire process including original inoculum, propagation,.. Master Batch Record Definition Fda.
From tutore.org
Master Batch Record Template Master Batch Record Definition Fda (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. 211.186 master production and control records. the record shall include: (a) the master record file provides the complete. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and. (1) the name of the type a. Master Batch Record Definition Fda.
From pharmaceuticalsindex.com
Different Types of Batch Records in Pharmaceutical Industry Master Batch Record Definition Fda for drug products, a master batch record covering the entire process including original inoculum, propagation,. 211.186 master production and control records. (1) the name of the type a medicated article (s) and a specimen copy of its label. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. 225.102. Master Batch Record Definition Fda.
From www.cphi-online.com
Electronic Batch Manufacturing Record (eBMR) Samvardhana Motherson Master Batch Record Definition Fda the record shall include: (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. (1) the name of the type a medicated article (s) and a specimen copy of its label. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) to assure uniformity from batch. Master Batch Record Definition Fda.
From www.youtube.com
Master Batch Record Requirements Drug Development (Phase 1) YouTube Master Batch Record Definition Fda (a) the master record file provides the complete. the record shall include: (1) the name of the type a medicated article (s) and a specimen copy of its label. 225.102 master record file and production records. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. (a) to. Master Batch Record Definition Fda.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Master Batch Record Definition Fda a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. 225.102 master record file and production records. the record shall include: (a) to assure uniformity from batch to batch, master. (1) the name of the type a medicated article (s) and a specimen copy of its label. (a) the. Master Batch Record Definition Fda.
From www.youtube.com
GMP Case Study Master Batch Record and Labeling Errors YouTube Master Batch Record Definition Fda (a) the master record file provides the complete. (1) the name of the type a medicated article (s) and a specimen copy of its label. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and. . Master Batch Record Definition Fda.
From manoxblog.com
Master Electronic Batch Records InstantGMP™ M A N O X B L O G Master Batch Record Definition Fda the batch production record should be checked before issuance to ensure that it is the correct version and a legible. 211.186 master production and control records. (a) the master record file provides the complete. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and. for drug products, a master batch. Master Batch Record Definition Fda.
From es.scribd.com
Check List of Batch Record PDF Production And Manufacturing Master Batch Record Definition Fda (a) the master record file provides the complete. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. (1) the name of the type a medicated article (s) and a specimen copy of its label. 225.102 master record file and production records. (a) an accurate reproduction of the appropriate. Master Batch Record Definition Fda.
From www.plm.automation.siemens.com
What is a master batch record Siemens Software Master Batch Record Definition Fda (a) the master record file provides the complete. for drug products, a master batch record covering the entire process including original inoculum, propagation,. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. (1) the name of the type a medicated article (s) and a specimen copy of its. Master Batch Record Definition Fda.
From www.instantgmp.com
Device Master Record Master Batch Record Definition Fda the record shall include: 211.186 master production and control records. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) an accurate reproduction of the appropriate master production or control. Master Batch Record Definition Fda.
From pharmaguidelines.co.uk
Standard Operating Procedure Master Batch Record Creation Master Batch Record Definition Fda (1) the name of the type a medicated article (s) and a specimen copy of its label. 211.186 master production and control records. 225.102 master record file and production records. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) an accurate reproduction of the appropriate master production or control record, checked. Master Batch Record Definition Fda.
From mavink.com
Batch Production Record Templates Master Batch Record Definition Fda for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. (1) the name of the type a medicated article (s). Master Batch Record Definition Fda.
From ciqa.net
What is a Master Batch Record (MBR) Versus a Batch Record (BR). Master Batch Record Definition Fda a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (1) the name of the type a medicated. Master Batch Record Definition Fda.
From academy.qualitysystems.it
Il Master Batch Record Academy QS Master Batch Record Definition Fda the record shall include: 225.102 master record file and production records. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. (a) to assure uniformity from batch to batch, master. (a) the master record file provides the complete. (a) an accurate reproduction of the appropriate master production. Master Batch Record Definition Fda.
From www.mastercontrol.com
Master Production Records MasterControl Master Batch Record Definition Fda the batch production record should be checked before issuance to ensure that it is the correct version and a legible. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and. (1). Master Batch Record Definition Fda.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Master Batch Record Definition Fda a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. 225.102 master record file and production records. (a) the master record file provides the complete. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. 211.186 master production and control records. for drug. Master Batch Record Definition Fda.
From weeverapps.com
Master Batch Records Weever Master Batch Record Definition Fda the record shall include: 211.186 master production and control records. 225.102 master record file and production records. (1) the name of the type a medicated article (s) and a specimen copy of its label. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and. (a) the master record file provides the. Master Batch Record Definition Fda.
From www.datacor.com
How to Prepare a Batch Manufacturing Record & Free Template (2022) Master Batch Record Definition Fda (1) the name of the type a medicated article (s) and a specimen copy of its label. (a) to assure uniformity from batch to batch, master. the record shall include: for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) an accurate reproduction of the appropriate master production or control record,. Master Batch Record Definition Fda.
From old.sermitsiaq.ag
Master Batch Record Template Master Batch Record Definition Fda a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. the record shall include: for drug products, a master batch record covering the entire process including original inoculum, propagation,. (1) the name of the type a medicated article (s) and a specimen copy of its label. (a) the. Master Batch Record Definition Fda.
From www.slideserve.com
PPT Current Good Manufacturing Practices (cGMP’s) PowerPoint Master Batch Record Definition Fda (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. the record shall include: 225.102 master record file and production records. (a) to assure uniformity from batch to batch, master. (a) the master record file provides the complete. for drug products, a master batch record covering the entire process including original inoculum,. Master Batch Record Definition Fda.
From www.datacor.com
2024 Batch Manufacturing Record Definition, Template & Examples Master Batch Record Definition Fda (1) the name of the type a medicated article (s) and a specimen copy of its label. (a) to assure uniformity from batch to batch, master. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. the record shall include: (a) an accurate reproduction of the appropriate. Master Batch Record Definition Fda.
From dxotibwbh.blob.core.windows.net
Master Batch Record Eudralex at Freda Fontaine blog Master Batch Record Definition Fda a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. the record. Master Batch Record Definition Fda.
From dxotibwbh.blob.core.windows.net
Master Batch Record Eudralex at Freda Fontaine blog Master Batch Record Definition Fda the record shall include: 225.102 master record file and production records. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and. a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. (a) the master record file provides the complete. (1) the. Master Batch Record Definition Fda.
From www.instantgmp.com
Batch Production Record InstantGMP Master Batch Record Definition Fda 211.186 master production and control records. 225.102 master record file and production records. (1) the name of the type a medicated article (s) and a specimen copy of its label. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. the batch production record should be checked before issuance to ensure that it. Master Batch Record Definition Fda.
From www.youtube.com
Batch Record ReviewWhat to Look For YouTube Master Batch Record Definition Fda (1) the name of the type a medicated article (s) and a specimen copy of its label. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and. 225.102 master record file and production records. for drug products, a master batch record covering the entire process including original inoculum, propagation,. a. Master Batch Record Definition Fda.
From www.mycellhub.com
7 key requirements for electronic batch records Master Batch Record Definition Fda the batch production record should be checked before issuance to ensure that it is the correct version and a legible. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) the master record file provides the. Master Batch Record Definition Fda.
From tech-publish.com
Standard Operating Procedure For Review Of Batch Records Techpublish Master Batch Record Definition Fda 211.186 master production and control records. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. (a) to assure uniformity from batch to batch, master. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. (a) the master record file provides the complete.. Master Batch Record Definition Fda.
From prntbl.concejomunicipaldechinu.gov.co
Master Batch Record Template prntbl.concejomunicipaldechinu.gov.co Master Batch Record Definition Fda (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy,. for drug products, a master batch record covering the entire process including original inoculum, propagation,. 225.102 master record file and production records. a master. Master Batch Record Definition Fda.
From manufacturing-software-blog.mrpeasy.com
How to Keep Perfect Batch Records? MRPeasy Master Batch Record Definition Fda a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. (a) the master record file provides the complete. (a) to assure uniformity from batch to batch, master. 211.186 master production and control records. (a) an accurate reproduction of the appropriate master production or control record, checked for accuracy, dated,. Master Batch Record Definition Fda.