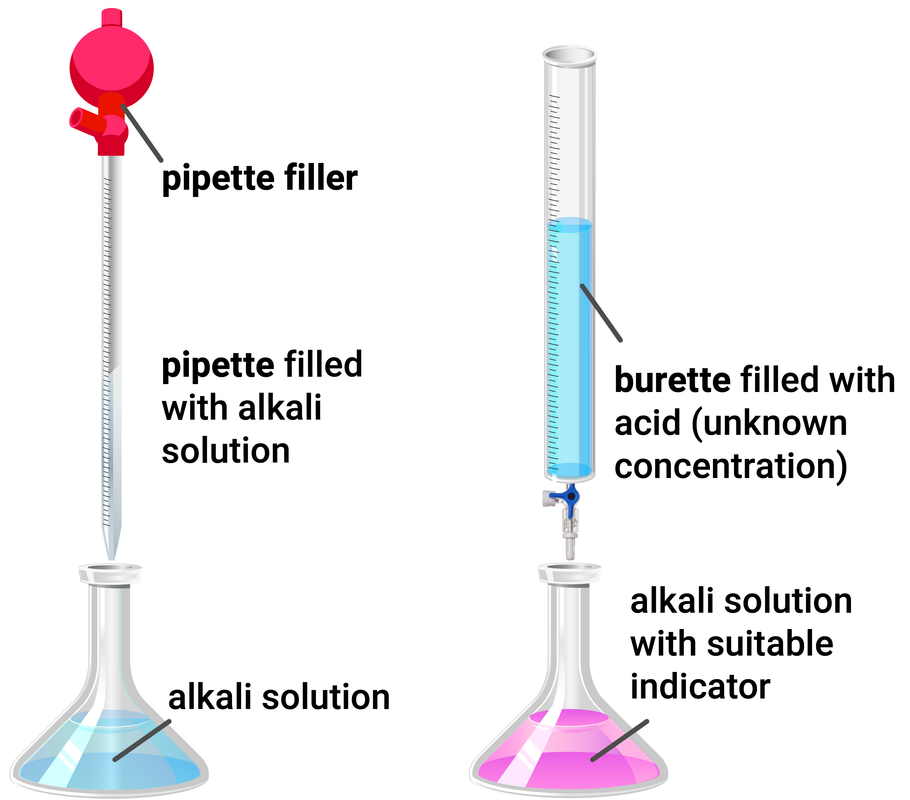
Discussion For Titration Experiment . write your conclusion. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration. In a titration, the conclusion is often a simple statement of the experimentally. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Volumetric procedures are among the most common and convenient. Titration is the process of determining the concentration of an unknown solution using a solution of known.
from revisechemistry.uk
a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Volumetric procedures are among the most common and convenient. In a titration, the conclusion is often a simple statement of the experimentally. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration. write your conclusion. Titration is the process of determining the concentration of an unknown solution using a solution of known. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to.
Titration OCR GCSE PAG 6 revisechemistry.uk
Discussion For Titration Experiment Titration is the process of determining the concentration of an unknown solution using a solution of known. In a titration, the conclusion is often a simple statement of the experimentally. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. Volumetric procedures are among the most common and convenient. write your conclusion. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titration is the process of determining the concentration of an unknown solution using a solution of known. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to.
From studylib.net
The Titration of Acetic Acid in Vinegar Discussion For Titration Experiment a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Volumetric procedures are among the most common and convenient. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point. Discussion For Titration Experiment.
From www.sciencephoto.com
Titration experiment Stock Image C010/9624 Science Photo Library Discussion For Titration Experiment a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. write your conclusion. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In this experiment you will be determining the volume of. Discussion For Titration Experiment.
From fity.club
Titration Experiment Discussion For Titration Experiment Volumetric procedures are among the most common and convenient. In a titration, the conclusion is often a simple statement of the experimentally. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. a titration is an experiment where a volume of a solution. Discussion For Titration Experiment.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Discussion For Titration Experiment In this experiment you will be determining the volume of sodium hydroxide solution of known concentration. write your conclusion. Titration is the process of determining the concentration of an unknown solution using a solution of known. In a titration, the conclusion is often a simple statement of the experimentally. In a ph titration you measure the ph as a. Discussion For Titration Experiment.
From www.studocu.com
Lecture Notes 9 + Experiment 9 ACIDBASE TITRATION Experiment 9 Discussion For Titration Experiment in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titration is the process of determining the concentration of an unknown solution using a solution of known. Volumetric procedures are among the most common and convenient. In a ph titration you measure the ph as a function of the volume. Discussion For Titration Experiment.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Discussion For Titration Experiment Volumetric procedures are among the most common and convenient. In a titration, the conclusion is often a simple statement of the experimentally. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. in this experiment students neutralise sodium hydroxide with hydrochloric acid to. Discussion For Titration Experiment.
From www.youtube.com
Titration of Oxalic Acid vs NaOH oxalic acid vs NaOH titration Discussion For Titration Experiment Titration is the process of determining the concentration of an unknown solution using a solution of known. Volumetric procedures are among the most common and convenient. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. a titration is an experiment where a volume of a solution of known. Discussion For Titration Experiment.
From fity.club
Titration Discussion For Titration Experiment a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration. a titration is an experiment where a volume of a solution of known concentration is added to. Discussion For Titration Experiment.
From www.science-revision.co.uk
Titrations Discussion For Titration Experiment In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. In a titration, the conclusion is often a simple statement of the experimentally. write your conclusion. a titration is an experiment where a volume of a solution of known concentration is added. Discussion For Titration Experiment.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Discussion For Titration Experiment write your conclusion. Titration is the process of determining the concentration of an unknown solution using a solution of known. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In this experiment you will be determining the volume of sodium hydroxide solution. Discussion For Titration Experiment.
From www.sciencephoto.com
Titration experiment Stock Image C010/9622 Science Photo Library Discussion For Titration Experiment Titration is the process of determining the concentration of an unknown solution using a solution of known. Volumetric procedures are among the most common and convenient. write your conclusion. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In a titration, the. Discussion For Titration Experiment.
From letitsnowglobe.co.uk
Titration procedure pdf Discussion For Titration Experiment In this experiment you will be determining the volume of sodium hydroxide solution of known concentration. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. a titration is an experiment where a volume of a solution of known concentration is added to. Discussion For Titration Experiment.
From www.microlit.com
An Advanced Guide to Titration Microlit Discussion For Titration Experiment in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Volumetric procedures are among the most common and convenient. write your conclusion. In. Discussion For Titration Experiment.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Discussion For Titration Experiment Volumetric procedures are among the most common and convenient. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. Titration is the process of determining the concentration of an unknown solution using a solution of known. a titration is an experiment where a. Discussion For Titration Experiment.
From acidsandbasesandmore.weebly.com
Titrations CHEMISTRY 101 Discussion For Titration Experiment in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. In this experiment you will be determining the volume of sodium hydroxide solution of. Discussion For Titration Experiment.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Discussion For Titration Experiment Titration is the process of determining the concentration of an unknown solution using a solution of known. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. Volumetric procedures are among the most common and convenient. In this experiment you will be determining the. Discussion For Titration Experiment.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Discussion For Titration Experiment a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Volumetric procedures are among the most common and convenient. Titration is the process of determining the concentration of an unknown solution using a solution of known. in this experiment students neutralise sodium hydroxide. Discussion For Titration Experiment.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Discussion For Titration Experiment Titration is the process of determining the concentration of an unknown solution using a solution of known. write your conclusion. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. a titration is an experiment where a volume of a solution of known concentration is added to a. Discussion For Titration Experiment.
From letitsnowglobe.co.uk
Titration procedure pdf Discussion For Titration Experiment write your conclusion. Volumetric procedures are among the most common and convenient. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. Titration is the process of determining the concentration of an unknown solution using a solution of known. a titration is. Discussion For Titration Experiment.
From www.youtube.com
Titration Experiment & Calculate the Molarity of Acetic Acid in Vinegar Discussion For Titration Experiment write your conclusion. In a titration, the conclusion is often a simple statement of the experimentally. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. Volumetric procedures are among the most common and convenient. a titration is an experiment where a. Discussion For Titration Experiment.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Discussion For Titration Experiment Volumetric procedures are among the most common and convenient. write your conclusion. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In a titration, the conclusion. Discussion For Titration Experiment.
From theedge.com.hk
Chemistry How To Titration The Edge Discussion For Titration Experiment In a titration, the conclusion is often a simple statement of the experimentally. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In. Discussion For Titration Experiment.
From chemistry.analia-sanchez.net
Titration Notes Chemistry Classes / Ronald Reagan S.H.S. Discussion For Titration Experiment In a titration, the conclusion is often a simple statement of the experimentally. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. write your conclusion. Titration is the process of determining the concentration of an unknown solution using a solution of known.. Discussion For Titration Experiment.
From www.chemicals.co.uk
How To Carry Out a Titration Experiment The Chemistry Blog Discussion For Titration Experiment Volumetric procedures are among the most common and convenient. In a titration, the conclusion is often a simple statement of the experimentally. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In a. Discussion For Titration Experiment.
From mungfali.com
Titration Steps Discussion For Titration Experiment a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In a titration, the conclusion is often a simple statement of the experimentally. Volumetric procedures are among the most common and convenient. In a ph titration you measure the ph as a function of. Discussion For Titration Experiment.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Discussion For Titration Experiment In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration. Volumetric procedures are among the most common and convenient. a titration is an experiment where a volume. Discussion For Titration Experiment.
From stock.adobe.com
Acid base titration experiment and phases of color change during Discussion For Titration Experiment In a titration, the conclusion is often a simple statement of the experimentally. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Titration is the process of determining the concentration of an unknown solution using a solution of known. in this experiment. Discussion For Titration Experiment.
From www.studocu.com
Experiment 7 Titrations Experiment 7 Titrations Required reading Discussion For Titration Experiment In a titration, the conclusion is often a simple statement of the experimentally. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration. In a ph titration you measure the ph as a function. Discussion For Titration Experiment.
From olympiapublishers.com
Acid Base Titration Experiment Report Discussion For Titration Experiment in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. a titration is an experiment where a volume of a solution of known. Discussion For Titration Experiment.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Discussion For Titration Experiment in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titration is the process of determining the concentration of an unknown solution using a solution of known. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution. Discussion For Titration Experiment.
From igcse-chemistry-edexcel.blogspot.com
IGCSE Chemistry 4.9 describe experiments to carry out acidalkali Discussion For Titration Experiment a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In a titration, the conclusion is often a simple statement of the experimentally. a titration is an experiment where a volume of a solution of known concentration is added to a volume of. Discussion For Titration Experiment.
From brogan-blogmckenzie.blogspot.com
Acid Base Titration Experiment Report Discussion For Titration Experiment a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. write your conclusion. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. in this experiment. Discussion For Titration Experiment.
From letitsnowglobe.co.uk
Titration procedure pdf Discussion For Titration Experiment In this experiment you will be determining the volume of sodium hydroxide solution of known concentration. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Volumetric procedures are among the most common and convenient. Titration is the process of determining the concentration of. Discussion For Titration Experiment.
From revisechemistry.uk
Titration OCR GCSE PAG 6 revisechemistry.uk Discussion For Titration Experiment write your conclusion. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. Volumetric procedures are among the most common and convenient. Titration is the process of determining the concentration of an unknown solution using a solution of known. a titration is. Discussion For Titration Experiment.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Discussion For Titration Experiment a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. write your conclusion. Titration is the process of determining the concentration of an unknown solution using a solution of known. a titration is an experiment where a volume of a solution of. Discussion For Titration Experiment.