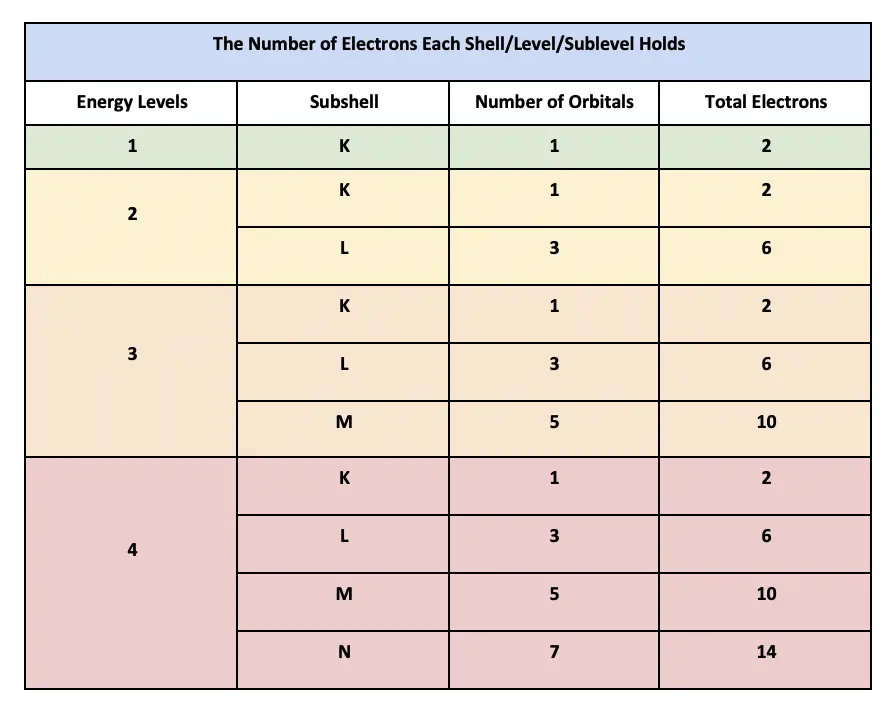
How Many Electrons Can Bromine Hold . To accommodate more than eight electrons,. The first shell can hold up to two electrons, the second shell can hold up to. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. 119 rows each shell can contain only a fixed number of electrons: Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. The third shell can carry up 18 electrons, but it is more stable by carrying only. Although the first element in this region is la, the filling of f orbitals starts from cerium (ce), and the elements together with it in the period 6 are called lanthanides (or rare earth elements). Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. The electron configuration of the bromine shows that there are two electrons in the k shell, eight in. The octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons.
from icdsc.org
119 rows each shell can contain only a fixed number of electrons: The first shell can carry up to two electrons, the second shell can carry up to eight electrons. The third shell can carry up 18 electrons, but it is more stable by carrying only. The octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. Although the first element in this region is la, the filling of f orbitals starts from cerium (ce), and the elements together with it in the period 6 are called lanthanides (or rare earth elements). The electron configuration of the bromine shows that there are two electrons in the k shell, eight in. To accommodate more than eight electrons,. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. The first shell can hold up to two electrons, the second shell can hold up to. Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus.
How many electrons are in each shell including 3p orbitals
How Many Electrons Can Bromine Hold The octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. To accommodate more than eight electrons,. Although the first element in this region is la, the filling of f orbitals starts from cerium (ce), and the elements together with it in the period 6 are called lanthanides (or rare earth elements). Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. The third shell can carry up 18 electrons, but it is more stable by carrying only. The first shell can hold up to two electrons, the second shell can hold up to. 119 rows each shell can contain only a fixed number of electrons: The octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. The electron configuration of the bromine shows that there are two electrons in the k shell, eight in.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram How Many Electrons Can Bromine Hold Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. The first. How Many Electrons Can Bromine Hold.
From poppykaur.z13.web.core.windows.net
How Many Electrons Does Bromine Have How Many Electrons Can Bromine Hold Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. The third shell can carry up 18 electrons, but it is more stable by carrying only. Although the first element in this region is. How Many Electrons Can Bromine Hold.
From www.slideserve.com
PPT Topic Chemistry Aim Explain how elements are classified in How Many Electrons Can Bromine Hold 119 rows each shell can contain only a fixed number of electrons: To accommodate more than eight electrons,. Although the first element in this region is la, the filling of f orbitals starts from cerium (ce), and the elements together with it in the period 6 are called lanthanides (or rare earth elements). The first shell can hold up to. How Many Electrons Can Bromine Hold.
From www.animalia-life.club
Electron Configuration For Bromine How Many Electrons Can Bromine Hold 119 rows each shell can contain only a fixed number of electrons: Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. The electron configuration of the bromine shows that there are two electrons in the k shell, eight in. Bromine has an atomic number of 35, which means that its. How Many Electrons Can Bromine Hold.
From ar.inspiredpencil.com
Orbital Diagram Of Bromine How Many Electrons Can Bromine Hold The first shell can hold up to two electrons, the second shell can hold up to. The electron configuration of the bromine shows that there are two electrons in the k shell, eight in. 119 rows each shell can contain only a fixed number of electrons: Although the first element in this region is la, the filling of f orbitals. How Many Electrons Can Bromine Hold.
From stock.adobe.com
Br Bromine Element Information Facts, Properties, Trends, Uses and How Many Electrons Can Bromine Hold Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. To accommodate more than eight electrons,. The octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. 119 rows each shell can contain only a fixed. How Many Electrons Can Bromine Hold.
From iperiodictable.com
How Can We Find A Electron Configuration For Bromine (Br) How Many Electrons Can Bromine Hold 119 rows each shell can contain only a fixed number of electrons: Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. Although the first element in this region is. How Many Electrons Can Bromine Hold.
From www.gauthmath.com
Solved How many protons, electron and neutron does bromine ion have How Many Electrons Can Bromine Hold The electron configuration of the bromine shows that there are two electrons in the k shell, eight in. Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. 119 rows each shell can contain only a fixed number of electrons: Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that. How Many Electrons Can Bromine Hold.
From www.vrogue.co
Bromine Electron Configuration Br With Orbital Diagra vrogue.co How Many Electrons Can Bromine Hold The octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. 119 rows each shell can contain only a fixed number of electrons: The third shell. How Many Electrons Can Bromine Hold.
From autoctrls.com
Understanding the Bromine Electron Dot Diagram A Comprehensive Guide How Many Electrons Can Bromine Hold 119 rows each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can hold up to. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. Bromine has an atomic number of 35, which means that its. How Many Electrons Can Bromine Hold.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements How Many Electrons Can Bromine Hold Although the first element in this region is la, the filling of f orbitals starts from cerium (ce), and the elements together with it in the period 6 are called lanthanides (or rare earth elements). 119 rows each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can. How Many Electrons Can Bromine Hold.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector How Many Electrons Can Bromine Hold 119 rows each shell can contain only a fixed number of electrons: Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. The third shell can carry up 18 electrons, but it is more stable by carrying only. The octet rule is based on the fact that each valence orbital (typically,. How Many Electrons Can Bromine Hold.
From chemwiki.ucdavis.edu
Electronic Configurations Chemwiki How Many Electrons Can Bromine Hold The first shell can hold up to two electrons, the second shell can hold up to. 119 rows each shell can contain only a fixed number of electrons: Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. The first shell can carry up to two electrons, the second. How Many Electrons Can Bromine Hold.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear How Many Electrons Can Bromine Hold Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. Although the first element in this region is la, the filling of f orbitals starts from cerium (ce), and the elements together with it in the period 6 are called lanthanides (or rare earth elements). The first shell can hold up. How Many Electrons Can Bromine Hold.
From slideplayer.com
Ionic and Covalent bonding Chapters 15 and ppt download How Many Electrons Can Bromine Hold 119 rows each shell can contain only a fixed number of electrons: Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. The electron configuration of the bromine shows that. How Many Electrons Can Bromine Hold.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? How Many Electrons Can Bromine Hold The first shell can hold up to two electrons, the second shell can hold up to. The octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. 119 rows each shell can contain only a fixed number of electrons: Electron configuration of bromine (br) is [ar] 3d¹⁰. How Many Electrons Can Bromine Hold.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID6667703 How Many Electrons Can Bromine Hold Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. The first shell can hold up to two electrons, the second shell can hold up to. The electron configuration of the. How Many Electrons Can Bromine Hold.
From www.youtube.com
How many valence electrons does bromine have? YouTube How Many Electrons Can Bromine Hold Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. The third shell can carry up 18 electrons, but it is more stable by carrying only. Electron configuration of bromine (br) is [ar] 3d¹⁰. How Many Electrons Can Bromine Hold.
From mungfali.com
Aufbau Diagram For Bromine How Many Electrons Can Bromine Hold Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. The electron configuration of the bromine shows that there are two electrons in the k shell, eight in. The third shell can carry up 18 electrons, but it is more stable by carrying only. 119 rows each shell can. How Many Electrons Can Bromine Hold.
From ar.inspiredpencil.com
Bromine Valence Electrons How Many Electrons Can Bromine Hold The first shell can carry up to two electrons, the second shell can carry up to eight electrons. The electron configuration of the bromine shows that there are two electrons in the k shell, eight in. The third shell can carry up 18 electrons, but it is more stable by carrying only. The octet rule is based on the fact. How Many Electrons Can Bromine Hold.
From periodictable.me
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram How Many Electrons Can Bromine Hold To accommodate more than eight electrons,. Although the first element in this region is la, the filling of f orbitals starts from cerium (ce), and the elements together with it in the period 6 are called lanthanides (or rare earth elements). The third shell can carry up 18 electrons, but it is more stable by carrying only. 119 rows each. How Many Electrons Can Bromine Hold.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron How Many Electrons Can Bromine Hold Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. The octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. The electron configuration of the bromine shows that there are two electrons in the k shell, eight in.. How Many Electrons Can Bromine Hold.
From www.animalia-life.club
Electron Configuration For Bromine How Many Electrons Can Bromine Hold Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. 119 rows each shell can contain only a fixed number of electrons: To accommodate more than eight electrons,. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. Bromine has. How Many Electrons Can Bromine Hold.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy How Many Electrons Can Bromine Hold Although the first element in this region is la, the filling of f orbitals starts from cerium (ce), and the elements together with it in the period 6 are called lanthanides (or rare earth elements). Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. The electron configuration of the bromine shows. How Many Electrons Can Bromine Hold.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) How Many Electrons Can Bromine Hold The electron configuration of the bromine shows that there are two electrons in the k shell, eight in. Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. The first shell can carry. How Many Electrons Can Bromine Hold.
From material-properties.org
Bromine Periodic Table and Atomic Properties How Many Electrons Can Bromine Hold Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. Although the first element in this region is la, the filling of f orbitals starts from cerium (ce), and the elements together with it in the period 6 are called lanthanides (or rare earth elements). The electron configuration of the bromine. How Many Electrons Can Bromine Hold.
From www.slideshare.net
Bromine berry How Many Electrons Can Bromine Hold The electron configuration of the bromine shows that there are two electrons in the k shell, eight in. The first shell can hold up to two electrons, the second shell can hold up to. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. 119 rows each shell can. How Many Electrons Can Bromine Hold.
From www.animalia-life.club
Electron Configuration For Bromine How Many Electrons Can Bromine Hold Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. The third shell can carry up 18 electrons, but it is more stable by carrying only. Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. The first shell can carry. How Many Electrons Can Bromine Hold.
From chemistryatomrevision.weebly.com
Electron Configuration Atomic Structure How Many Electrons Can Bromine Hold The electron configuration of the bromine shows that there are two electrons in the k shell, eight in. Although the first element in this region is la, the filling of f orbitals starts from cerium (ce), and the elements together with it in the period 6 are called lanthanides (or rare earth elements). The first shell can hold up to. How Many Electrons Can Bromine Hold.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic How Many Electrons Can Bromine Hold The first shell can carry up to two electrons, the second shell can carry up to eight electrons. The electron configuration of the bromine shows that there are two electrons in the k shell, eight in. Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. The octet rule is based. How Many Electrons Can Bromine Hold.
From icdsc.org
How many electrons are in each shell including 3p orbitals How Many Electrons Can Bromine Hold Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. The octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two. How Many Electrons Can Bromine Hold.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry How Many Electrons Can Bromine Hold Electron configuration of bromine (br) is [ar] 3d¹⁰ 4s² 4p⁵, indicating that br has 35 electrons distributed among its atomic orbitals. The electron configuration of the bromine shows that there are two electrons in the k shell, eight in. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Each orbital can. How Many Electrons Can Bromine Hold.
From www.animalia-life.club
Electron Configuration For Bromine How Many Electrons Can Bromine Hold Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. 119 rows each shell can contain only a fixed number of electrons: The third shell can carry up 18 electrons, but it is more stable by carrying only. The electron configuration of the bromine shows that there are two electrons in. How Many Electrons Can Bromine Hold.
From www.slideserve.com
PPT Quantum Number and Electron Configurations PowerPoint How Many Electrons Can Bromine Hold Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. The octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. 119 rows each shell can contain only a fixed number of electrons: The third shell can carry up. How Many Electrons Can Bromine Hold.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine How Many Electrons Can Bromine Hold The first shell can hold up to two electrons, the second shell can hold up to. The electron configuration of the bromine shows that there are two electrons in the k shell, eight in. Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel. The octet rule is based on the. How Many Electrons Can Bromine Hold.