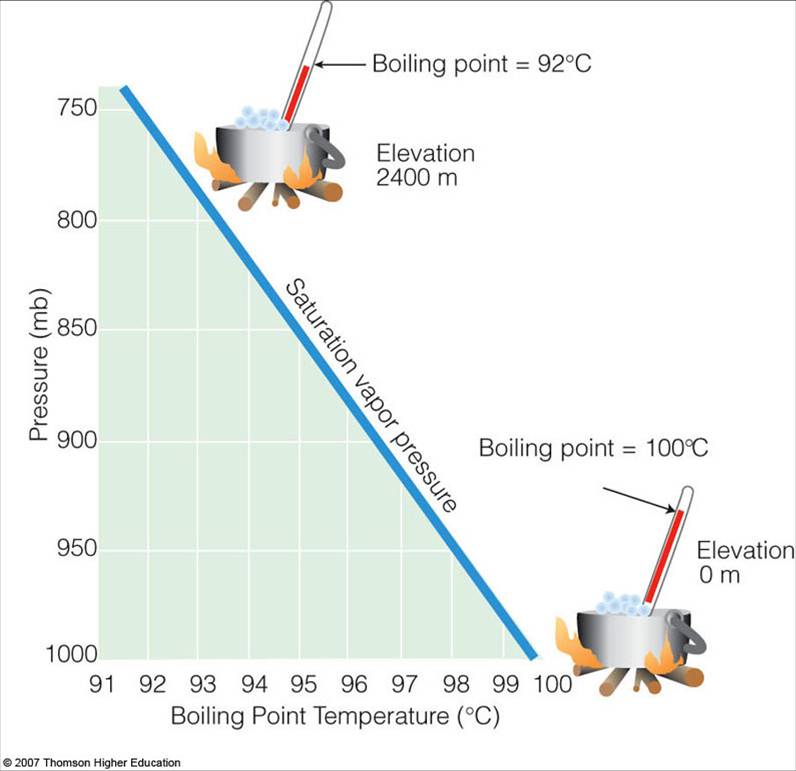
Does Increased Pressure Raise Boiling Point . The intermolecular forces increase with increasing polarization of bonds. Yes, the increase in pressure increases the boiling point of a liquid. This is the boiling point which is usually quoted in chemical literature. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. Boiling point increases with molecular weight, and with surface area. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). Normally when we boil a liquid, we do so at atmospheric pressure. As elevation increases, atmospheric pressure decreases because air is less dense at higher. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The opposite would also be true; When the vapour pressure equals atmospheric pressure, the liquid boils.
from apollo.lsc.vsc.edu
The intermolecular forces increase with increasing polarization of bonds. This is the boiling point which is usually quoted in chemical literature. When the vapour pressure equals atmospheric pressure, the liquid boils. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Yes, the increase in pressure increases the boiling point of a liquid. The opposite would also be true; Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. Boiling point increases with molecular weight, and with surface area. As elevation increases, atmospheric pressure decreases because air is less dense at higher.
Saturation Vapor Pressure and the Boiling Point
Does Increased Pressure Raise Boiling Point Normally when we boil a liquid, we do so at atmospheric pressure. Boiling point increases with molecular weight, and with surface area. The opposite would also be true; The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. When the vapour pressure equals atmospheric pressure, the liquid boils. Normally when we boil a liquid, we do so at atmospheric pressure. The intermolecular forces increase with increasing polarization of bonds. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). This is the boiling point which is usually quoted in chemical literature. Yes, the increase in pressure increases the boiling point of a liquid. As elevation increases, atmospheric pressure decreases because air is less dense at higher. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the.
From lambdageeks.com
Boiling Point and Pressure Relation, Impact, Effect, How And Why Does Increased Pressure Raise Boiling Point When the vapour pressure equals atmospheric pressure, the liquid boils. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Normally when we boil a liquid, we do so at atmospheric pressure. For example, an increase in pressure from 100 to 200. Does Increased Pressure Raise Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart Does Increased Pressure Raise Boiling Point Boiling point increases with molecular weight, and with surface area. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. The intermolecular forces increase with increasing polarization of bonds. The opposite would also be true; Boiling point of a liquid can be increased by increasing the external pressure on. Does Increased Pressure Raise Boiling Point.
From hxegzypap.blob.core.windows.net
Does Pressure Increase The Boiling Point at Edwin Dixon blog Does Increased Pressure Raise Boiling Point This is the boiling point which is usually quoted in chemical literature. Normally when we boil a liquid, we do so at atmospheric pressure. As elevation increases, atmospheric pressure decreases because air is less dense at higher. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. The opposite. Does Increased Pressure Raise Boiling Point.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills Does Increased Pressure Raise Boiling Point The intermolecular forces increase with increasing polarization of bonds. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. This is the boiling point which is usually quoted in chemical literature. Normally when we boil a liquid, we do so at atmospheric pressure. As elevation increases, atmospheric pressure decreases. Does Increased Pressure Raise Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Does Increased Pressure Raise Boiling Point Boiling point increases with molecular weight, and with surface area. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. The intermolecular forces increase with increasing polarization of bonds. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be. Does Increased Pressure Raise Boiling Point.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 Does Increased Pressure Raise Boiling Point When the vapour pressure equals atmospheric pressure, the liquid boils. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. As elevation increases, atmospheric pressure decreases because air is less dense at higher. For example, an increase in pressure from 100 to 200 mmhg results in an increase in. Does Increased Pressure Raise Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart Does Increased Pressure Raise Boiling Point When the vapour pressure equals atmospheric pressure, the liquid boils. The opposite would also be true; This is the boiling point which is usually quoted in chemical literature. As elevation increases, atmospheric pressure decreases because air is less dense at higher. The intermolecular forces increase with increasing polarization of bonds. Normally when we boil a liquid, we do so at. Does Increased Pressure Raise Boiling Point.
From byjus.com
How does external pressure affect the boiling point Does Increased Pressure Raise Boiling Point For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). This is the boiling point which is usually quoted in chemical literature. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion.. Does Increased Pressure Raise Boiling Point.
From facts.net
20 Fascinating Facts About Boiling Point Elevation Does Increased Pressure Raise Boiling Point If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The opposite would also be true; Normally when we boil a liquid, we do so at atmospheric pressure. Boiling point increases with molecular weight, and with surface area. This is the boiling. Does Increased Pressure Raise Boiling Point.
From pdfslide.net
(PDF) 73...the boiling point to rise. Effect of pressure on boiling and Does Increased Pressure Raise Boiling Point As elevation increases, atmospheric pressure decreases because air is less dense at higher. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. Yes, the increase in pressure increases the boiling point of a liquid. This is the boiling point which is usually quoted in chemical literature. The opposite. Does Increased Pressure Raise Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Does Increased Pressure Raise Boiling Point For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). Boiling point increases with molecular weight, and with surface area. Yes, the increase in pressure increases the boiling point of a liquid. The strength of intermolecular forces (and therefore impact on boiling points). Does Increased Pressure Raise Boiling Point.
From chem.libretexts.org
13.8 FreezingPoint Depression and BoilingPoint Elevation of Does Increased Pressure Raise Boiling Point Normally when we boil a liquid, we do so at atmospheric pressure. The opposite would also be true; The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. This is the boiling point which is usually quoted in chemical literature. The intermolecular forces increase with increasing polarization of bonds.. Does Increased Pressure Raise Boiling Point.
From pakmcqs.com
With the increase of pressure, the boiling point of any substance Does Increased Pressure Raise Boiling Point Yes, the increase in pressure increases the boiling point of a liquid. Boiling point increases with molecular weight, and with surface area. Normally when we boil a liquid, we do so at atmospheric pressure. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to. Does Increased Pressure Raise Boiling Point.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Does Increased Pressure Raise Boiling Point Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. Yes, the increase in pressure increases the boiling point of a liquid. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from. Does Increased Pressure Raise Boiling Point.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free Does Increased Pressure Raise Boiling Point As elevation increases, atmospheric pressure decreases because air is less dense at higher. Normally when we boil a liquid, we do so at atmospheric pressure. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. The opposite would also be true; If this pressure is the standard pressure of. Does Increased Pressure Raise Boiling Point.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point Does Increased Pressure Raise Boiling Point For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). When the vapour pressure equals atmospheric pressure, the liquid boils. The intermolecular forces increase with increasing polarization of bonds. The strength of intermolecular forces (and therefore impact on boiling points) is ionic >. Does Increased Pressure Raise Boiling Point.
From www.slideserve.com
PPT Energy, Temperature, Phase Changes PowerPoint Does Increased Pressure Raise Boiling Point The opposite would also be true; Normally when we boil a liquid, we do so at atmospheric pressure. This is the boiling point which is usually quoted in chemical literature. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). As elevation increases,. Does Increased Pressure Raise Boiling Point.
From www.youtube.com
Boiling point and external pressure YouTube Does Increased Pressure Raise Boiling Point Normally when we boil a liquid, we do so at atmospheric pressure. The intermolecular forces increase with increasing polarization of bonds. As elevation increases, atmospheric pressure decreases because air is less dense at higher. Boiling point increases with molecular weight, and with surface area. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at. Does Increased Pressure Raise Boiling Point.
From slideplayer.com
Chapter 9 Heat. ppt download Does Increased Pressure Raise Boiling Point The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature. Does Increased Pressure Raise Boiling Point.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Does Increased Pressure Raise Boiling Point The opposite would also be true; The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. This is the boiling point which is usually quoted in chemical literature. Yes, the increase in pressure increases the boiling point of a liquid. Boiling point increases with molecular weight, and with surface. Does Increased Pressure Raise Boiling Point.
From ponasa.condesan-ecoandes.org
Water Boiling Point Vs Pressure Chart Ponasa Does Increased Pressure Raise Boiling Point Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. The intermolecular forces increase with increasing polarization of bonds. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. The opposite would also be true;. Does Increased Pressure Raise Boiling Point.
From www.pinterest.com
Mastering Pressure Cooking Understanding Boiling Point at Different Does Increased Pressure Raise Boiling Point Normally when we boil a liquid, we do so at atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The intermolecular forces increase with increasing polarization of bonds. When the vapour pressure equals atmospheric pressure, the liquid boils. The. Does Increased Pressure Raise Boiling Point.
From mungfali.com
Water Boiling Point Pressure Chart Does Increased Pressure Raise Boiling Point Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. This is the boiling point which is usually quoted in chemical literature. Yes, the increase in pressure increases the boiling point of a liquid. When the vapour pressure equals atmospheric pressure, the liquid boils. For example,. Does Increased Pressure Raise Boiling Point.
From mungfali.com
Vapor Pressure And Boiling Point Relationship Does Increased Pressure Raise Boiling Point The opposite would also be true; Yes, the increase in pressure increases the boiling point of a liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. When the vapour pressure equals atmospheric pressure, the liquid boils. As elevation increases, atmospheric. Does Increased Pressure Raise Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart Does Increased Pressure Raise Boiling Point Normally when we boil a liquid, we do so at atmospheric pressure. The opposite would also be true; The intermolecular forces increase with increasing polarization of bonds. Boiling point increases with molecular weight, and with surface area. Yes, the increase in pressure increases the boiling point of a liquid. For example, an increase in pressure from 100 to 200 mmhg. Does Increased Pressure Raise Boiling Point.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Does Increased Pressure Raise Boiling Point Yes, the increase in pressure increases the boiling point of a liquid. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. Normally when we boil a liquid, we do so at atmospheric pressure. Boiling point increases with molecular weight, and with surface area. If this. Does Increased Pressure Raise Boiling Point.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Does Increased Pressure Raise Boiling Point Normally when we boil a liquid, we do so at atmospheric pressure. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. Boiling point increases with molecular weight, and with surface area. The intermolecular forces increase with increasing polarization of bonds. Yes, the increase in pressure increases the boiling. Does Increased Pressure Raise Boiling Point.
From www.worksheetsplanet.com
What is Boiling Point Does Increased Pressure Raise Boiling Point As elevation increases, atmospheric pressure decreases because air is less dense at higher. When the vapour pressure equals atmospheric pressure, the liquid boils. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. Yes, the increase in pressure increases the boiling point of a liquid. If this pressure is. Does Increased Pressure Raise Boiling Point.
From mungfali.com
Water Boiling Point Pressure Chart Does Increased Pressure Raise Boiling Point For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). This is the boiling point which is usually quoted in chemical literature. Yes, the increase in pressure increases the boiling point of a liquid. As elevation increases, atmospheric pressure decreases because air is. Does Increased Pressure Raise Boiling Point.
From slideplayer.com
How to Use This Presentation ppt download Does Increased Pressure Raise Boiling Point If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Yes, the increase in pressure increases the boiling point of a liquid. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of. Does Increased Pressure Raise Boiling Point.
From eatingrules.com
The Science of Pressure Cookers Eating Rules Does Increased Pressure Raise Boiling Point The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. For example, an increase in pressure from 100 to 200 mmhg. Does Increased Pressure Raise Boiling Point.
From www.vrogue.co
Physical Equilibrium Vapor Pressure And Boiling Point vrogue.co Does Increased Pressure Raise Boiling Point When the vapour pressure equals atmospheric pressure, the liquid boils. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. The opposite would also be true; Yes, the increase in pressure increases the boiling point of a liquid. Boiling point increases with molecular weight, and with. Does Increased Pressure Raise Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Does Increased Pressure Raise Boiling Point For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). The intermolecular forces increase with increasing polarization of bonds. This is the boiling point which is usually quoted in chemical literature. When the vapour pressure equals atmospheric pressure, the liquid boils. As elevation. Does Increased Pressure Raise Boiling Point.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point Does Increased Pressure Raise Boiling Point If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). When the vapour pressure equals. Does Increased Pressure Raise Boiling Point.
From diagramfricanofc.z21.web.core.windows.net
Initial Boiling Point And Final Boiling Point Does Increased Pressure Raise Boiling Point This is the boiling point which is usually quoted in chemical literature. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. The opposite would also be true; The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole. Does Increased Pressure Raise Boiling Point.