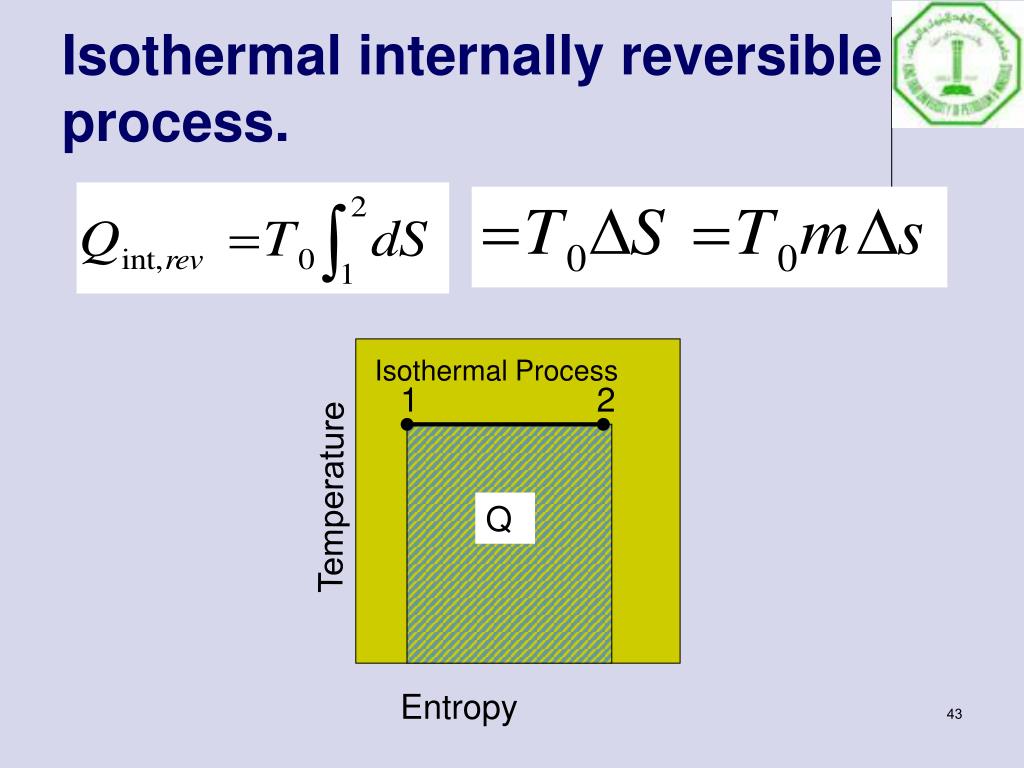
Heat Transfer Isothermal Process Reversible . The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. Consider a system at temperature t in thermal. An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process. Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature difference is.
from www.slideserve.com
An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature difference is. The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a. Consider a system at temperature t in thermal. A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process.
PPT Second Law of Thermodynamics PowerPoint Presentation, free
Heat Transfer Isothermal Process Reversible Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process. Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature difference is. The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a. An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. Consider a system at temperature t in thermal.
From www.slideserve.com
PPT Reversible Process PowerPoint Presentation, free download ID527789 Heat Transfer Isothermal Process Reversible A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. An important feature of a reversible process is that, depending on the process, it represents the maximum work that. Heat Transfer Isothermal Process Reversible.
From www.youtube.com
ISOTHERMAL PROCESSCONSTANT TEMPERATURE PROCESS THERMODYNAMIC Heat Transfer Isothermal Process Reversible The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. A reversible process in which energy is exchanged. Heat Transfer Isothermal Process Reversible.
From study.com
Determining the Work Done by an Isothermal Process. Chemistry Heat Transfer Isothermal Process Reversible The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a. Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature difference is. A thermodynamic process is called reversible if an. Heat Transfer Isothermal Process Reversible.
From www.slideserve.com
PPT Energy Transfer by Heat, Work and Mass PowerPoint Presentation Heat Transfer Isothermal Process Reversible A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. An important feature of a reversible process is that, depending on the process, it represents the maximum work that. Heat Transfer Isothermal Process Reversible.
From www.slideserve.com
PPT Reversible Process PowerPoint Presentation, free download ID527789 Heat Transfer Isothermal Process Reversible An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature difference is. A reversible process in which energy is exchanged via heat between a finite system and. Heat Transfer Isothermal Process Reversible.
From pressbooks.bccampus.ca
6.5 Entropy and entropy generation Introduction to Engineering Heat Transfer Isothermal Process Reversible Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature. Heat Transfer Isothermal Process Reversible.
From www.eigenplus.com
Isothermal process Definition, Work done & Explanation eigenplus Heat Transfer Isothermal Process Reversible A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process. Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature difference is. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. The. Heat Transfer Isothermal Process Reversible.
From www.slideserve.com
PPT Reversible Process PowerPoint Presentation, free download ID527789 Heat Transfer Isothermal Process Reversible An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible. Heat Transfer Isothermal Process Reversible.
From www.thoughtco.com
What is an Isothermal Process in Physics? Heat Transfer Isothermal Process Reversible Consider a system at temperature t in thermal. Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature difference is. An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. A reversible process in which energy is. Heat Transfer Isothermal Process Reversible.
From www.youtube.com
Thermodynamic Processes Isobaric, Isochoric, Isothermal and Adiabatic Heat Transfer Isothermal Process Reversible An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process. Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature. Heat Transfer Isothermal Process Reversible.
From www.slideserve.com
PPT Second Law of Thermodynamics PowerPoint Presentation, free Heat Transfer Isothermal Process Reversible Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process. Consider a system at temperature t in thermal. The second law of thermodynamics states that in a reversible process, the entropy of. Heat Transfer Isothermal Process Reversible.
From www.slideserve.com
PPT Objectives PowerPoint Presentation, free download ID2250016 Heat Transfer Isothermal Process Reversible A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process. Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature difference is. The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible. Heat Transfer Isothermal Process Reversible.
From tipseri.com
What is a reversible isothermal process? Tipseri Heat Transfer Isothermal Process Reversible A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process. An important. Heat Transfer Isothermal Process Reversible.
From www.vrogue.co
Thermodynamic Processes Types And Equations Chemtalk vrogue.co Heat Transfer Isothermal Process Reversible An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. Consider a system at temperature t in thermal. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. A thermodynamic process is called reversible if. Heat Transfer Isothermal Process Reversible.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID3150253 Heat Transfer Isothermal Process Reversible A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. Consider a system at temperature t in thermal. Noting that heat transfer does not occur without a temperature difference. Heat Transfer Isothermal Process Reversible.
From www.youtube.com
Isothermal process Thermodynamics Work, Heat & Internal Energy, PV Heat Transfer Isothermal Process Reversible A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a. Noting that heat transfer does not occur without a. Heat Transfer Isothermal Process Reversible.
From www.tec-science.com
Isothermal process in a closed system tecscience Heat Transfer Isothermal Process Reversible A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. Consider a system at temperature t in thermal. The second law of thermodynamics states that in a reversible process,. Heat Transfer Isothermal Process Reversible.
From eduinput.com
Isothermal Process Isothermal Process and Boyle’s Law Heat Transfer Isothermal Process Reversible A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process. The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process,. Heat Transfer Isothermal Process Reversible.
From www.eigenplus.com
Isothermal process Definition, Work done & Explanation eigenplus Heat Transfer Isothermal Process Reversible The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a. A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process. Both isothermal and adiabatic processes sketched on a pv graph (discussed in. Heat Transfer Isothermal Process Reversible.
From ppt-online.org
Laws of Thermodynamics презентация онлайн Heat Transfer Isothermal Process Reversible A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. Consider a system at temperature t in thermal. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. The second law of thermodynamics states that in a reversible process,. Heat Transfer Isothermal Process Reversible.
From diagramlibrarygermins.z19.web.core.windows.net
Isothermal Pv Diagram Heat Transfer Isothermal Process Reversible Consider a system at temperature t in thermal. Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature difference is. The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a.. Heat Transfer Isothermal Process Reversible.
From www.tec-science.com
Isothermal process in a closed system tecscience Heat Transfer Isothermal Process Reversible An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process. A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must. Heat Transfer Isothermal Process Reversible.
From www.doubtnut.com
The PV diagram shows four different possible paths of a reversible pro Heat Transfer Isothermal Process Reversible Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature difference is. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. An important feature of a reversible process is that, depending on the process, it represents the maximum work. Heat Transfer Isothermal Process Reversible.
From www.slideserve.com
PPT Second Year Chemistry PowerPoint Presentation, free download ID Heat Transfer Isothermal Process Reversible The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a. An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. Noting that heat transfer does. Heat Transfer Isothermal Process Reversible.
From forums.studentdoctor.net
Isothermal process Student Doctor Network Heat Transfer Isothermal Process Reversible The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. An important feature of a reversible process is. Heat Transfer Isothermal Process Reversible.
From www.youtube.com
Ideal Isothermal Process on Piston find Total Work or Heat Added Heat Transfer Isothermal Process Reversible An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process. Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature. Heat Transfer Isothermal Process Reversible.
From nigerianscholars.com
Entropy and the Second Law of Thermodynamics Thermodynamics Heat Transfer Isothermal Process Reversible An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. Consider a system at temperature t in thermal. The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of. Heat Transfer Isothermal Process Reversible.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID3150253 Heat Transfer Isothermal Process Reversible A thermodynamic process is called reversible if an infinitesimal change of the external condition reverses the process. An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas. Heat Transfer Isothermal Process Reversible.
From criticalthinking.cloud
second law of thermodynamics solved problems pdf Heat Transfer Isothermal Process Reversible A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. Noting that heat transfer does not occur without a temperature difference and that heat transfer across. Heat Transfer Isothermal Process Reversible.
From slideplayer.com
Chapter Seven Entropy ppt download Heat Transfer Isothermal Process Reversible A reversible process in which energy is exchanged via heat between a finite system and an infinite reservoir must be. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas. Heat Transfer Isothermal Process Reversible.
From www.tf.uni-kiel.de
Carnot cycle pV diagram and TS diagram Heat Transfer Isothermal Process Reversible Consider a system at temperature t in thermal. The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a. Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle.. Heat Transfer Isothermal Process Reversible.
From mech-engineeringbd.blogspot.com
Isothermal Process (Constant Temperature Process) Mechanical Engineering Heat Transfer Isothermal Process Reversible Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature difference is. The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a. Both isothermal and adiabatic processes sketched on a. Heat Transfer Isothermal Process Reversible.
From www.slideserve.com
PPT 18. Heat, Work, & First Law of Thermodynamics PowerPoint Heat Transfer Isothermal Process Reversible The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a. Noting that heat transfer does not occur without a temperature difference and that heat transfer across a finite temperature difference is. An important feature of a reversible process is. Heat Transfer Isothermal Process Reversible.
From chemistnotes.com
Thermodynamic process Isothermal, Isobaric, Isochoric, Adiabatic, and Heat Transfer Isothermal Process Reversible Both isothermal and adiabatic processes sketched on a pv graph (discussed in the first law of thermodynamics) are reversible in principle. An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be extracted in going. Consider a system at temperature t in thermal. A reversible process in which energy is. Heat Transfer Isothermal Process Reversible.
From www.toppr.com
Consider the reversible isothermal exapnsion of an ideal gas in a Heat Transfer Isothermal Process Reversible Consider a system at temperature t in thermal. The second law of thermodynamics states that in a reversible process, the entropy of the universe is constant, whereas in an irreversible process, such as the transfer of heat from a. An important feature of a reversible process is that, depending on the process, it represents the maximum work that can be. Heat Transfer Isothermal Process Reversible.