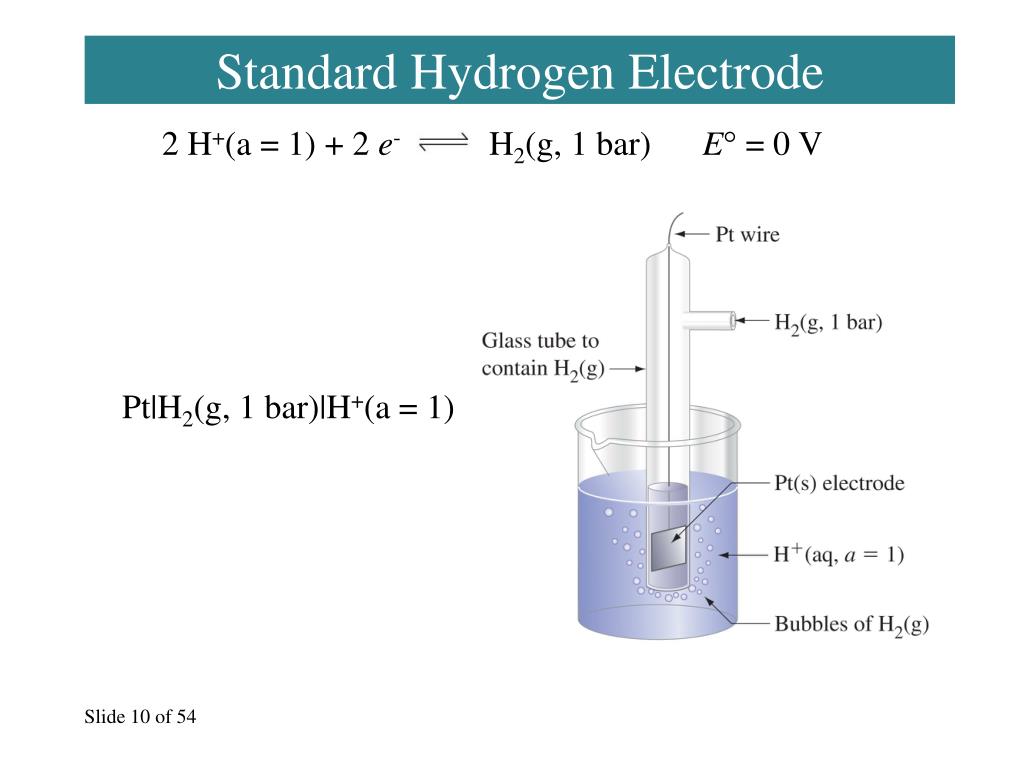
Explain Standard Hydrogen Electrode With Labelled Diagram . Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. Hydrogen gas in equilibrium with h + ions of. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. A hydrogen electrode in which the pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution. The standard hydrogen electrode is defined as having. See a labelled diagram of she and a solved example.
from www.slideserve.com
Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. See a labelled diagram of she and a solved example. A hydrogen electrode in which the pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. Hydrogen gas in equilibrium with h + ions of. The standard hydrogen electrode is defined as having.
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free download ID5581588
Explain Standard Hydrogen Electrode With Labelled Diagram Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. See a labelled diagram of she and a solved example. Hydrogen gas in equilibrium with h + ions of. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. A hydrogen electrode in which the pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. The standard hydrogen electrode is defined as having.
From brainly.in
what is standard hydrogen electrode draw Brainly.in Explain Standard Hydrogen Electrode With Labelled Diagram Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. See a labelled diagram of she and a solved example. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. Hydrogen gas in equilibrium with h +. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.meritnation.com
Explain construction and working of standard Hydrogen electrode Chemistry Electrochemistry Explain Standard Hydrogen Electrode With Labelled Diagram Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. A hydrogen electrode in which the pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution. See a labelled diagram of she and a solved example.. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.slideserve.com
PPT Electroanalysis PowerPoint Presentation, free download ID820788 Explain Standard Hydrogen Electrode With Labelled Diagram See a labelled diagram of she and a solved example. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. The standard hydrogen electrode is defined as having. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Hydrogen gas in. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.sciencephoto.com
Hydrogen electrode apparatus Stock Image C011/4570 Science Photo Library Explain Standard Hydrogen Electrode With Labelled Diagram Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. See a labelled diagram of she and a solved example. Learn how to use. Explain Standard Hydrogen Electrode With Labelled Diagram.
From exofdjhjw.blob.core.windows.net
What Is Standard Hydrogen Electrode Explain With Diagram at Tamiko Sanders blog Explain Standard Hydrogen Electrode With Labelled Diagram Hydrogen gas in equilibrium with h + ions of. See a labelled diagram of she and a solved example. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. The standard hydrogen electrode is defined as having. Learn about the standard hydrogen electrode (she), a. Explain Standard Hydrogen Electrode With Labelled Diagram.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12, Electro Chemistry Explain Standard Hydrogen Electrode With Labelled Diagram Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. See a labelled diagram of she and a solved example. The standard hydrogen electrode is defined as having. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.youtube.com
20.10 calculating emf with a standard hydrogen electrode YouTube Explain Standard Hydrogen Electrode With Labelled Diagram See a labelled diagram of she and a solved example. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. The standard hydrogen electrode is defined as having. A hydrogen electrode in which the pressure of hydrogen gas is maintained at 1 atm and the concentration of h +. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Explain Standard Hydrogen Electrode With Labelled Diagram Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. Hydrogen gas in equilibrium with h + ions of. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.youtube.com
DAT Reaction at Standard Hydrogen Electrode Explained YouTube Explain Standard Hydrogen Electrode With Labelled Diagram Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Hydrogen gas in equilibrium with h + ions of. The standard hydrogen electrode is defined as having. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. See a labelled diagram. Explain Standard Hydrogen Electrode With Labelled Diagram.
From brainly.in
What is standard hydrogen electrode? How is it prepared? Explain with labelled diagram? Brainly.in Explain Standard Hydrogen Electrode With Labelled Diagram See a labelled diagram of she and a solved example. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. The standard hydrogen electrode is defined as having. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h +. Explain Standard Hydrogen Electrode With Labelled Diagram.
From gaskatel.de
Usage of the hydrogen electrodes HydroFlex and MiniHydroFlex Gaskatel Explain Standard Hydrogen Electrode With Labelled Diagram See a labelled diagram of she and a solved example. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. A hydrogen electrode in which the pressure of. Explain Standard Hydrogen Electrode With Labelled Diagram.
From app.pandai.org
Standard Electrode Potential Explain Standard Hydrogen Electrode With Labelled Diagram Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. See a labelled diagram of she and a solved example. Hydrogen gas in equilibrium with h + ions of. The standard. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.vrogue.co
Ii Draw Well Labeled Diagram Of Standard Hydrogen Ele vrogue.co Explain Standard Hydrogen Electrode With Labelled Diagram Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. The standard hydrogen electrode is defined as having. A hydrogen electrode in which the pressure of hydrogen gas is maintained at. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.vrogue.co
Ii Draw Well Labeled Diagram Of Standard Hydrogen Ele vrogue.co Explain Standard Hydrogen Electrode With Labelled Diagram The standard hydrogen electrode is defined as having. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.vectorstock.com
Diagram representation of the element hydrogen Vector Image Explain Standard Hydrogen Electrode With Labelled Diagram Hydrogen gas in equilibrium with h + ions of. The standard hydrogen electrode is defined as having. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells.. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.slideserve.com
PPT CHEM1612 Pharmacy Week 9 Nernst Equation PowerPoint Presentation ID6272423 Explain Standard Hydrogen Electrode With Labelled Diagram Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. See a labelled diagram of she and a solved example. Hydrogen gas in equilibrium with h + ions of. The standard hydrogen electrode is defined as having. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen. Explain Standard Hydrogen Electrode With Labelled Diagram.
From sites.google.com
Electrochemistry engineering chemistry Explain Standard Hydrogen Electrode With Labelled Diagram The standard hydrogen electrode is defined as having. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. See a labelled diagram of she. Explain Standard Hydrogen Electrode With Labelled Diagram.
From wordwall.net
Standard hydrogen electrode Labelled diagram Explain Standard Hydrogen Electrode With Labelled Diagram Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. A hydrogen electrode in which the pressure of hydrogen gas is maintained at 1 atm and the concentration. Explain Standard Hydrogen Electrode With Labelled Diagram.
From sulekharanirxiichemistry.blogspot.com
SULEKHA Class XII Chemistry STANDARD HYDROGEN ELECTRODE Explain Standard Hydrogen Electrode With Labelled Diagram Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. See a labelled diagram of she and a solved example. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. The standard hydrogen electrode is defined as having. A hydrogen electrode. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free download ID5581588 Explain Standard Hydrogen Electrode With Labelled Diagram Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. See a labelled diagram of she and a solved example. Learn how to use. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free download ID350050 Explain Standard Hydrogen Electrode With Labelled Diagram Hydrogen gas in equilibrium with h + ions of. See a labelled diagram of she and a solved example. The standard hydrogen electrode is defined as having. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.slideserve.com
PPT Ch. 18 Electrochemistry PowerPoint Presentation, free download ID2921005 Explain Standard Hydrogen Electrode With Labelled Diagram Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. Hydrogen gas in equilibrium with h + ions of. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. Learn how to use the standard hydrogen electrode to. Explain Standard Hydrogen Electrode With Labelled Diagram.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Diagram Explain Standard Hydrogen Electrode With Labelled Diagram See a labelled diagram of she and a solved example. Hydrogen gas in equilibrium with h + ions of. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID541675 Explain Standard Hydrogen Electrode With Labelled Diagram Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. The standard hydrogen electrode is defined as having. See a labelled diagram of she and a solved example. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.vrogue.co
Draw Neat Labelled Diagram Of Standard Hydrogen Elect vrogue.co Explain Standard Hydrogen Electrode With Labelled Diagram Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Hydrogen gas in equilibrium with h + ions of. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. Learn what a standard hydrogen electrode (she) is, how it is constructed,. Explain Standard Hydrogen Electrode With Labelled Diagram.
From mungfali.com
Ppt Chapter 17 Electrochemistry Powerpoint Presentation, Free A12 Explain Standard Hydrogen Electrode With Labelled Diagram A hydrogen electrode in which the pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution. Hydrogen gas in equilibrium with h + ions of. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. The standard hydrogen electrode is. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.studypool.com
SOLUTION Standard hydrogen electrode,Definition,explanation. Studypool Explain Standard Hydrogen Electrode With Labelled Diagram The standard hydrogen electrode is defined as having. See a labelled diagram of she and a solved example. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode. Standard hydrogen electrode,. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Explain Standard Hydrogen Electrode With Labelled Diagram Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. The standard hydrogen electrode is defined as having. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Learn about the standard hydrogen electrode (she), a reference. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.slideserve.com
PPT CHEM 160 General Chemistry II Lecture Presentation Electrochemistry PowerPoint Explain Standard Hydrogen Electrode With Labelled Diagram Hydrogen gas in equilibrium with h + ions of. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. Learn what a standard hydrogen. Explain Standard Hydrogen Electrode With Labelled Diagram.
From issuu.com
standard hydrogen electrode by norainihamzah9 Issuu Explain Standard Hydrogen Electrode With Labelled Diagram Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. The standard hydrogen electrode is defined as having. Hydrogen gas in equilibrium with h + ions of. A hydrogen electrode in which the pressure of hydrogen gas is maintained at 1 atm and the concentration. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5744606 Explain Standard Hydrogen Electrode With Labelled Diagram Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Hydrogen gas in equilibrium with h + ions of. See a labelled diagram of she and a solved example. The standard. Explain Standard Hydrogen Electrode With Labelled Diagram.
From brainly.in
What is standard hydrogen electrode? how is it prepared? Explain with labeled diagram. Brainly.in Explain Standard Hydrogen Electrode With Labelled Diagram See a labelled diagram of she and a solved example. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. A hydrogen electrode in which the pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution. Learn how to use. Explain Standard Hydrogen Electrode With Labelled Diagram.
From stock.adobe.com
Standard hydrogen electrode diagram. Scientific vector illustration isolated on white background Explain Standard Hydrogen Electrode With Labelled Diagram The standard hydrogen electrode is defined as having. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. Hydrogen gas in equilibrium with h + ions of. A hydrogen electrode in which the pressure of hydrogen gas is maintained at 1 atm and the concentration. Explain Standard Hydrogen Electrode With Labelled Diagram.
From es.vecteezy.com
un estándar hidrógeno electrodo ella es un electrodo ese científicos utilizar como un referencia Explain Standard Hydrogen Electrode With Labelled Diagram Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. The standard hydrogen electrode is defined as having. See a labelled diagram of she and a solved example. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h +. Explain Standard Hydrogen Electrode With Labelled Diagram.
From www.linstitute.net
IB DP Chemistry HL复习笔记19.1.2 The Standard Hydrogen Electrode翰林国际教育 Explain Standard Hydrogen Electrode With Labelled Diagram Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration (h + ion) in a solution. See a labelled diagram of she and a solved example. Learn about the standard hydrogen electrode (she), a reference electrode with zero potential, and how it works as both anode and. A hydrogen electrode in. Explain Standard Hydrogen Electrode With Labelled Diagram.