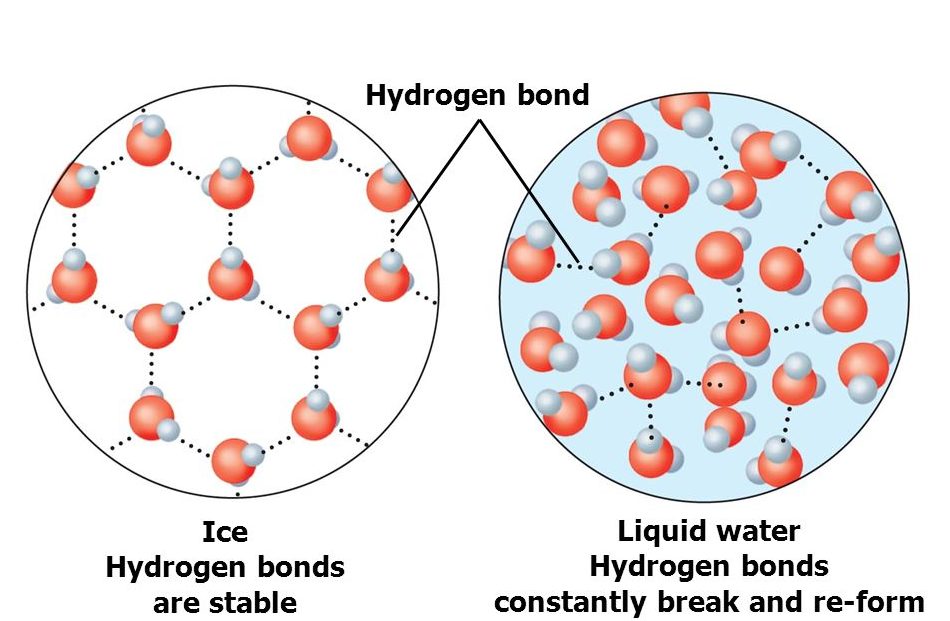
Why Is Ice Lighter Than Water Hydrogen Bonding . Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. As you (should) know, water is made up of one oxygen and two hydrogen atoms. When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the. In liquid form, as the molecules move around,. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. Most of the ice on earth's surface forms repeating hexagonal crystals. Ponds or lakes begin to freeze at the surface, closer to the cold air. These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at all). Ice is less dense than liquid water and so it floats. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. The reason why ice is less dense than water has to do with hydrogen bonds.
from www.hesco-mpl.com
Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. Ponds or lakes begin to freeze at the surface, closer to the cold air. In liquid form, as the molecules move around,. The reason why ice is less dense than water has to do with hydrogen bonds. When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the. Ice is less dense than liquid water and so it floats. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. As you (should) know, water is made up of one oxygen and two hydrogen atoms. These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at all). The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms.
为什么冰会浮在水面上?——万博网页版Techiescientist 万博网页版,万博体育app手机版登录
Why Is Ice Lighter Than Water Hydrogen Bonding As you (should) know, water is made up of one oxygen and two hydrogen atoms. Ice is less dense than liquid water and so it floats. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Ponds or lakes begin to freeze at the surface, closer to the cold air. These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at all). When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the. Most of the ice on earth's surface forms repeating hexagonal crystals. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. The reason why ice is less dense than water has to do with hydrogen bonds. As you (should) know, water is made up of one oxygen and two hydrogen atoms. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. In liquid form, as the molecules move around,.
From www.thesciencehive.co.uk
Alcohols* — the science sauce Why Is Ice Lighter Than Water Hydrogen Bonding The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Ice is less dense than liquid water and so it floats. As you (should) know, water is made up of one oxygen and two hydrogen atoms. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water. Why Is Ice Lighter Than Water Hydrogen Bonding.
From slideplayer.com
Matter is made of elements ppt download Why Is Ice Lighter Than Water Hydrogen Bonding The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at all). In liquid form, as the molecules move. Why Is Ice Lighter Than Water Hydrogen Bonding.
From sciencenotes.org
Hydrogen Bond Definition and Examples Why Is Ice Lighter Than Water Hydrogen Bonding As you (should) know, water is made up of one oxygen and two hydrogen atoms. Ponds or lakes begin to freeze at the surface, closer to the cold air. When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the. When water freezes, these hydrogen bonds form a. Why Is Ice Lighter Than Water Hydrogen Bonding.
From chamotgallery.com
Hydrogen Bonding Definition, Types, Effects and Properties (2022) Why Is Ice Lighter Than Water Hydrogen Bonding Ice is less dense than liquid water and so it floats. Ponds or lakes begin to freeze at the surface, closer to the cold air. As you (should) know, water is made up of one oxygen and two hydrogen atoms. Most of the ice on earth's surface forms repeating hexagonal crystals. These interactions are called hydrogen bonds (which, technically speaking,. Why Is Ice Lighter Than Water Hydrogen Bonding.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Why Is Ice Lighter Than Water Hydrogen Bonding When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the. In liquid form, as the molecules move around,. The reason why ice is less dense than water has to do with hydrogen bonds. The molecules in ice are held further apart by the hydrogen bonds between the. Why Is Ice Lighter Than Water Hydrogen Bonding.
From askfilo.com
7. Why is ice at 273 K more effective in cooling than water at the same t.. Why Is Ice Lighter Than Water Hydrogen Bonding Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. Ice is less dense than liquid water and so it floats. Most of the ice on earth's surface forms repeating hexagonal crystals. In liquid. Why Is Ice Lighter Than Water Hydrogen Bonding.
From www.doubtnut.com
Why is ice less dense than water and what kind of attractive forces mu Why Is Ice Lighter Than Water Hydrogen Bonding Most of the ice on earth's surface forms repeating hexagonal crystals. As you (should) know, water is made up of one oxygen and two hydrogen atoms. Ponds or lakes begin to freeze at the surface, closer to the cold air. In liquid form, as the molecules move around,. These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at. Why Is Ice Lighter Than Water Hydrogen Bonding.
From johnnyholland.org
Why is Ice Less Dense Than Water? Johnny Holland Why Is Ice Lighter Than Water Hydrogen Bonding When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. Ponds or lakes begin to freeze at the surface, closer to the cold air. When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the. Most of the ice on earth's surface forms repeating. Why Is Ice Lighter Than Water Hydrogen Bonding.
From mungfali.com
Ice Hydrogen Bonding Why Is Ice Lighter Than Water Hydrogen Bonding Most of the ice on earth's surface forms repeating hexagonal crystals. The reason why ice is less dense than water has to do with hydrogen bonds. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. When water solidifies, hydrogen bonding between the molecules forces the molecules to. Why Is Ice Lighter Than Water Hydrogen Bonding.
From answeringallthings.com
Why is ice less dense than water hydrogen bonding? answeringallthings/ Why Is Ice Lighter Than Water Hydrogen Bonding Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at all). The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When water freezes, these hydrogen bonds form. Why Is Ice Lighter Than Water Hydrogen Bonding.
From www.worldatlas.com
Do Water And Ice Weigh The Same? WorldAtlas Why Is Ice Lighter Than Water Hydrogen Bonding As you (should) know, water is made up of one oxygen and two hydrogen atoms. Ponds or lakes begin to freeze at the surface, closer to the cold air. In liquid form, as the molecules move around,. These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at all). Since hydrogen bonds are the primary intermolecular forces in h2o,. Why Is Ice Lighter Than Water Hydrogen Bonding.
From www.scienceabc.com
Why Do Fingers/Hands Stick To Ice? » ScienceABC Why Is Ice Lighter Than Water Hydrogen Bonding These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at all). The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Ponds or lakes begin to freeze at the surface, closer to the cold air. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. When. Why Is Ice Lighter Than Water Hydrogen Bonding.
From www.teachoo.com
Liquids generally have lower density as compared to solids. But you Why Is Ice Lighter Than Water Hydrogen Bonding As you (should) know, water is made up of one oxygen and two hydrogen atoms. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. Ice is less dense than liquid water and so it floats. These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at. Why Is Ice Lighter Than Water Hydrogen Bonding.
From www.slideserve.com
PPT Hydrogen bonding PowerPoint Presentation ID2452680 Why Is Ice Lighter Than Water Hydrogen Bonding These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at all). Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Ice is less dense than liquid water. Why Is Ice Lighter Than Water Hydrogen Bonding.
From socratic.org
A sample of water has a fixed volume and shape. What state is it in? Socratic Why Is Ice Lighter Than Water Hydrogen Bonding When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. The. Why Is Ice Lighter Than Water Hydrogen Bonding.
From exyczdtof.blob.core.windows.net
Why Is Ice Lighter Than Liquid Water at Juan Poe blog Why Is Ice Lighter Than Water Hydrogen Bonding In liquid form, as the molecules move around,. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. Ponds or lakes begin to freeze at the surface, closer to the cold air. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. When water. Why Is Ice Lighter Than Water Hydrogen Bonding.
From chemistryskills.com
Hydrogen Bonding Chemistry Skills Why Is Ice Lighter Than Water Hydrogen Bonding Most of the ice on earth's surface forms repeating hexagonal crystals. Ponds or lakes begin to freeze at the surface, closer to the cold air. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are. Why Is Ice Lighter Than Water Hydrogen Bonding.
From zulfafiqah95.blogspot.com.es
Assalamualaikum.... Why Is Ice Lighter Than Water Hydrogen Bonding In liquid form, as the molecules move around,. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. Ice is less dense than liquid water and so it floats. When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the. Most of the ice. Why Is Ice Lighter Than Water Hydrogen Bonding.
From tinyhousegarage.com
Why Does Ice Float On Water Chemistry? Why Is Ice Lighter Than Water Hydrogen Bonding The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at all). Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid. Why Is Ice Lighter Than Water Hydrogen Bonding.
From answeringallthings.com
Why is ice lighter than water hydrogen bonding? answeringallthings/ Why Is Ice Lighter Than Water Hydrogen Bonding In liquid form, as the molecules move around,. The reason why ice is less dense than water has to do with hydrogen bonds. As you (should) know, water is made up of one oxygen and two hydrogen atoms. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. Ice is less dense than liquid water and. Why Is Ice Lighter Than Water Hydrogen Bonding.
From byjus.com
Solid ice is lighter than water .Explain Why Is Ice Lighter Than Water Hydrogen Bonding Ice is less dense than liquid water and so it floats. Most of the ice on earth's surface forms repeating hexagonal crystals. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. In liquid form, as the molecules move around,. When water solidifies, hydrogen bonding between the molecules. Why Is Ice Lighter Than Water Hydrogen Bonding.
From www.hesco-mpl.com
为什么冰会浮在水面上?——万博网页版Techiescientist 万博网页版,万博体育app手机版登录 Why Is Ice Lighter Than Water Hydrogen Bonding When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the. As you (should) know, water is made up of one oxygen and two hydrogen atoms. These interactions are called hydrogen bonds (which, technically. Why Is Ice Lighter Than Water Hydrogen Bonding.
From ar.inspiredpencil.com
Density Of Solids In Water Why Is Ice Lighter Than Water Hydrogen Bonding As you (should) know, water is made up of one oxygen and two hydrogen atoms. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the.. Why Is Ice Lighter Than Water Hydrogen Bonding.
From slideplayer.com
Chapter 15 “Water and Aqueous Systems” ppt download Why Is Ice Lighter Than Water Hydrogen Bonding The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. In liquid form, as the molecules move around,. These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at all). Ponds or lakes begin to freeze at the surface, closer to the cold air. The reason why ice is less dense. Why Is Ice Lighter Than Water Hydrogen Bonding.
From www.slideserve.com
PPT WATER, H 2 0 PowerPoint Presentation, free download ID1957755 Why Is Ice Lighter Than Water Hydrogen Bonding Most of the ice on earth's surface forms repeating hexagonal crystals. The reason why ice is less dense than water has to do with hydrogen bonds. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at all). Ponds or lakes. Why Is Ice Lighter Than Water Hydrogen Bonding.
From www.youtube.com
Why is ice less dense than water YouTube Why Is Ice Lighter Than Water Hydrogen Bonding As you (should) know, water is made up of one oxygen and two hydrogen atoms. In liquid form, as the molecules move around,. When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science.. Why Is Ice Lighter Than Water Hydrogen Bonding.
From animalia-life.club
Hydrogen Bonds Ice Why Is Ice Lighter Than Water Hydrogen Bonding When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. In liquid form, as the molecules move around,. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those. Why Is Ice Lighter Than Water Hydrogen Bonding.
From slideplayer.com
Chemistry Chapter 13, 14, and 15 Jeopardy ppt download Why Is Ice Lighter Than Water Hydrogen Bonding When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. Ice is less dense than liquid water and so it floats. As you (should) know, water is made up of one oxygen and two hydrogen atoms. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those. Why Is Ice Lighter Than Water Hydrogen Bonding.
From www.pinterest.com
Water Expansion When Freezing Science Facts Water molecule structure, Water molecule, The Why Is Ice Lighter Than Water Hydrogen Bonding When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. These interactions are called hydrogen. Why Is Ice Lighter Than Water Hydrogen Bonding.
From webmis.highland.cc.il.us
Intermolecular Forces Why Is Ice Lighter Than Water Hydrogen Bonding The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. Ice is less dense than liquid water and so it floats. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are. Why Is Ice Lighter Than Water Hydrogen Bonding.
From byjus.com
Why boiling point of water is more than hydrogen fluoride? Why Is Ice Lighter Than Water Hydrogen Bonding Most of the ice on earth's surface forms repeating hexagonal crystals. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the. Ice is less dense than liquid water and. Why Is Ice Lighter Than Water Hydrogen Bonding.
From www.ib.bioninja.com.au
Hydrogen Bonding Why Is Ice Lighter Than Water Hydrogen Bonding These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at all). When water solidifies, hydrogen bonding between the molecules forces the molecules to line up in a way that creates empty space between the. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. As you (should) know, water is. Why Is Ice Lighter Than Water Hydrogen Bonding.
From www.science-revision.co.uk
Hydrogen bonding Why Is Ice Lighter Than Water Hydrogen Bonding The reason why ice is less dense than water has to do with hydrogen bonds. Since hydrogen bonds are the primary intermolecular forces in h2o, the hydrogen bonds in liquid water are stronger than those in ice. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. The molecules in ice are held further apart by. Why Is Ice Lighter Than Water Hydrogen Bonding.
From juniorferslamb.blogspot.com
Why is Ice Less Dense Than Water Why Is Ice Lighter Than Water Hydrogen Bonding The reason why ice is less dense than water has to do with hydrogen bonds. When water freezes, these hydrogen bonds form a crystal lattice, minchew told live science. Ice is less dense than liquid water and so it floats. Most of the ice on earth's surface forms repeating hexagonal crystals. The molecules in ice are held further apart by. Why Is Ice Lighter Than Water Hydrogen Bonding.
From www.meritnation.com
Why hydrogen bond in ice is stronger than the hydrogen bond in water Please elaborate Why Is Ice Lighter Than Water Hydrogen Bonding The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Most of the ice on earth's surface forms repeating hexagonal crystals. Ice is less dense than liquid water and so it floats. Ponds or lakes begin to freeze at the surface, closer to the cold air. These interactions are called hydrogen bonds. Why Is Ice Lighter Than Water Hydrogen Bonding.