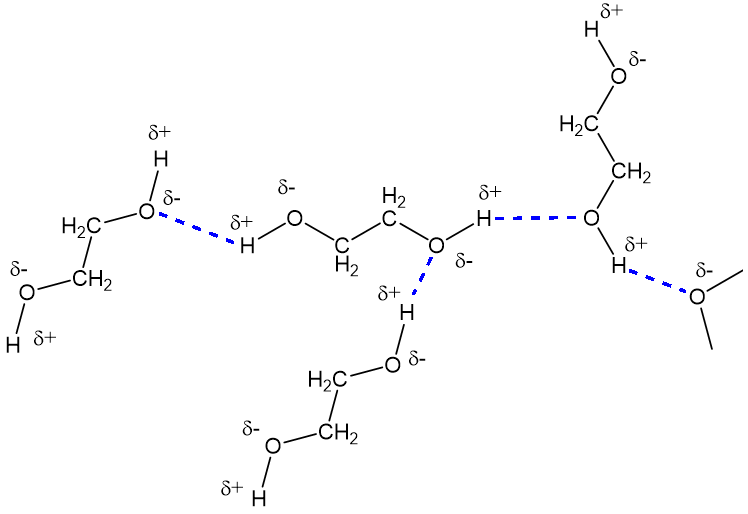
Ethylene Glycol In Water . Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Its most common use is as an automotive antifreeze. This property is exploited in some cosmetics,. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). It is obtained by the oxidation. It may also enter the environment from its uses in deicing airplane runways and from spills and. Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Ethylene glycol should be avoided if there is a slightest chance of leakage to. Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. A 1:1 solution of ethylene glycol and. Ethylene glycol may be discharged into wastewater from its production and use.
from www.chm.bris.ac.uk
Ethylene glycol may be discharged into wastewater from its production and use. Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. Its most common use is as an automotive antifreeze. It is obtained by the oxidation. Ethylene glycol should be avoided if there is a slightest chance of leakage to. It may also enter the environment from its uses in deicing airplane runways and from spills and. Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. This property is exploited in some cosmetics,. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). A 1:1 solution of ethylene glycol and.
Ethylene Glycol Molecule of the Month June 2018 (JSMol version)
Ethylene Glycol In Water Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. It is obtained by the oxidation. It may also enter the environment from its uses in deicing airplane runways and from spills and. Ethylene glycol should be avoided if there is a slightest chance of leakage to. Ethylene glycol may be discharged into wastewater from its production and use. Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Its most common use is as an automotive antifreeze. This property is exploited in some cosmetics,. A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere.
From northslopechillers.com
Glycol Chiller Systems (Where do they fit into YOUR process?) Ethylene Glycol In Water Ethylene glycol may be discharged into wastewater from its production and use. Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. This property is exploited in some cosmetics,. Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Ethylene glycol. Ethylene Glycol In Water.
From ar.inspiredpencil.com
Ethylene Glycol Structure Ethylene Glycol In Water Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. Its most common use is as an automotive antifreeze. It may also enter the environment from its uses in deicing airplane runways and from spills and. This property is exploited in some. Ethylene Glycol In Water.
From pediaa.com
Difference Between Ethylene Glycol and Propylene Glycol Definition Ethylene Glycol In Water Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. It is obtained by the oxidation. Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. Ethylene glycol should be avoided if there is a slightest chance of. Ethylene Glycol In Water.
From chempedia.info
Ethylene glycol, phase diagram Big Chemical Encyclopedia Ethylene Glycol In Water This property is exploited in some cosmetics,. Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. It may also enter the environment from its uses in deicing airplane runways and from spills and. Its most common use is as an automotive antifreeze. A 1:1 solution of ethylene glycol and. It is obtained by the oxidation.. Ethylene Glycol In Water.
From www.researchgate.net
Sea water/Ethylene Glycol solution heat exchanger. Download Ethylene Glycol In Water Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. It may also enter the environment from its uses in deicing airplane runways and from spills and. A 1:1 solution of ethylene glycol and. Ethylene glycol should be avoided if there is a slightest chance of leakage to. Learn about the chemical and physical properties of ethylene glycol/water mixtures,. Ethylene Glycol In Water.
From mavink.com
Ethylene Glycol Graph Ethylene Glycol In Water This property is exploited in some cosmetics,. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Its most common use is as an automotive antifreeze. Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. It is obtained by the oxidation. Ethylene glycol may be discharged into wastewater from its production. Ethylene Glycol In Water.
From www.numerade.com
SOLVEDFortyfive kilograms of a solution containing 30 wt ethylene Ethylene Glycol In Water It is obtained by the oxidation. A 1:1 solution of ethylene glycol and. Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. Ethylene glycol should be avoided if there is a slightest chance of leakage to. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Its most common use is as an automotive. Ethylene Glycol In Water.
From corecheminc.com
Ethylene Glycol / Water Mixture Properties CORECHEM Inc. Ethylene Glycol In Water Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Its most common use is as an automotive antifreeze. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol is ‘hygroscopic’ meaning it absorbs. Ethylene Glycol In Water.
From www.toppr.com
45 g of ethylene glycol (C2H6O2) is mixed with 600 g of water. The Ethylene Glycol In Water It may also enter the environment from its uses in deicing airplane runways and from spills and. Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol is ‘hygroscopic’ meaning it. Ethylene Glycol In Water.
From www.youtube.com
35 solution of ethylene glycol is used as antifreeze it lowers the Ethylene Glycol In Water Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. This property is exploited in some cosmetics,. Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. It may also enter the environment from its uses in deicing airplane runways and from spills and. Its most common use is as an automotive antifreeze. A 1:1. Ethylene Glycol In Water.
From hohwatertechnology.com
Closed Loop Water Treatment Managing Glycol Ethylene Glycol In Water Its most common use is as an automotive antifreeze. Ethylene glycol should be avoided if there is a slightest chance of leakage to. A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). This property is exploited in some cosmetics,. Ethylene glycol may be discharged into wastewater. Ethylene Glycol In Water.
From chempedia.info
Ethylene glycol in water Big Chemical Encyclopedia Ethylene Glycol In Water Ethylene glycol may be discharged into wastewater from its production and use. Its most common use is as an automotive antifreeze. Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. This property is exploited in some cosmetics,. Alcohols with two oh. Ethylene Glycol In Water.
From www.chm.bris.ac.uk
Ethylene Glycol Molecule of the Month June 2018 (JSMol version) Ethylene Glycol In Water Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. Ethylene glycol may be discharged into wastewater from its production and use. Its most common use is as an automotive antifreeze. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). It is obtained by the oxidation. It may also. Ethylene Glycol In Water.
From www.maratekenvironmental.com
What is Glycol, and How Can it be Used as a Solvent? Maratek Ethylene Glycol In Water Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. This property is exploited in some cosmetics,. It is obtained by the oxidation. A 1:1 solution of ethylene glycol and. It may also enter the environment from its uses in deicing airplane. Ethylene Glycol In Water.
From mavink.com
Ethylene Glycol Freeze Chart Ethylene Glycol In Water Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. Ethylene glycol may be discharged into wastewater from its production and use. This property is exploited in some cosmetics,. Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f).. Ethylene Glycol In Water.
From www.chegg.com
Obtain a formula to get the Viscosity of aqueous Ethylene Glycol In Water Its most common use is as an automotive antifreeze. Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Ethylene glycol may be discharged into wastewater from its production and use. Ethylene glycol should be avoided if there is a slightest chance of leakage to. Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere.. Ethylene Glycol In Water.
From www.youtube.com
45g of ethylene glycol (C2H6O2) is mixed with 600g of water. Calculate Ethylene Glycol In Water Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. A 1:1 solution of ethylene glycol and. This property is exploited in some cosmetics,. Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. Ethylene glycol may be discharged into wastewater from its production and use. Its most common use is as. Ethylene Glycol In Water.
From www.priyamstudycentre.com
Ethylene Glycol Properties, Formula, Structure, Production Ethylene Glycol In Water Its most common use is as an automotive antifreeze. Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. It is obtained by the oxidation. It may also enter the environment from its uses in deicing airplane runways and from spills and. A 1:1 solution of ethylene glycol and. Ethylene glycol should be avoided if there. Ethylene Glycol In Water.
From www.semanticscholar.org
[PDF] Ethylene Glycol and Its Mixtures with Water and Electrolytes Ethylene Glycol In Water Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Ethylene glycol may be discharged into wastewater from its production and use. Its most common use is as an automotive antifreeze. It is obtained by the oxidation. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene glycol is ‘hygroscopic’ meaning. Ethylene Glycol In Water.
From www.chegg.com
Liquidliquid extraction of ethylene glycol from Ethylene Glycol In Water Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. It may also enter the environment from its uses in deicing airplane runways and from spills and. Its most common use is as an automotive antifreeze. This property is exploited in some cosmetics,. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene glycol. Ethylene Glycol In Water.
From chempedia.info
Ethylene glycol, phase diagram Big Chemical Encyclopedia Ethylene Glycol In Water Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Its most common use is as an automotive antifreeze. Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. This property is exploited in some cosmetics,. It may also enter the environment from its uses in deicing airplane runways and from spills and.. Ethylene Glycol In Water.
From mungfali.com
Ethylene Glycol Density Chart Ethylene Glycol In Water It is obtained by the oxidation. Its most common use is as an automotive antifreeze. This property is exploited in some cosmetics,. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). It may also enter the environment from its uses in deicing airplane runways and from spills and. Ethylene glycol should be avoided. Ethylene Glycol In Water.
From mungfali.com
Ethylene Glycol Density Chart Ethylene Glycol In Water Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Its most common use is as an automotive antifreeze. A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol should be avoided if there is a slightest chance of leakage to.. Ethylene Glycol In Water.
From thermtest.com
Thermal Conductivity of Ethylene GlycolWater Mixtures Ethylene Glycol In Water It is obtained by the oxidation. Ethylene glycol may be discharged into wastewater from its production and use. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. Ethylene glycol should be avoided if there is a slightest chance of leakage to. A 1:1 solution. Ethylene Glycol In Water.
From www.numerade.com
SOLVED Water and antifrceze (ethylene glycol) mixtures have the Ethylene Glycol In Water Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Its most common use is as an automotive antifreeze. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol should be avoided if there. Ethylene Glycol In Water.
From www.vectorstock.com
Chemical formula and model ethylene glycol Vector Image Ethylene Glycol In Water A 1:1 solution of ethylene glycol and. Ethylene glycol may be discharged into wastewater from its production and use. It may also enter the environment from its uses in deicing airplane runways and from spills and. This property is exploited in some cosmetics,. Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. Ethylene glycol is. Ethylene Glycol In Water.
From www.ethersolvent.com
EP 5 Soluble In Water Ethylene Glycol Monopropyl Ether Most Organic Ethylene Glycol In Water Its most common use is as an automotive antifreeze. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol may be discharged into wastewater from its production and use. A 1:1 solution of ethylene glycol and. It may also enter the environment from its uses in deicing airplane runways and from spills. Ethylene Glycol In Water.
From www.chegg.com
Ethylene glycol and water are flash distilled in a Ethylene Glycol In Water A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. Its most common use is as an automotive antifreeze. It may also enter the environment from its uses in deicing airplane runways and from spills and.. Ethylene Glycol In Water.
From cqconcepts.com
Ethylene Glycol 99 1 Gallon Ethylene Glycol In Water It may also enter the environment from its uses in deicing airplane runways and from spills and. Its most common use is as an automotive antifreeze. Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Ethylene glycol should be avoided if there is a slightest chance of leakage to. Ethylene glycol is ‘hygroscopic’ meaning it. Ethylene Glycol In Water.
From www.toppr.com
45 g of ethylene glycol (C2H6O2) is mixed with 600 g of water. The Ethylene Glycol In Water Its most common use is as an automotive antifreeze. Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. It is obtained by the oxidation. It may also enter the environment from its uses in deicing airplane runways and from spills and. A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, sweet, slightly. Ethylene Glycol In Water.
From www.researchgate.net
The same volume (100 ml) of ethylene glycol solution (50... Download Ethylene Glycol In Water Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. This property is exploited in some cosmetics,. Its most common use is as an automotive antifreeze. It may also enter the environment from its uses in deicing airplane runways and from spills and. Ethylene glycol. Ethylene Glycol In Water.
From mungfali.com
Ethylene Glycol Density Chart Ethylene Glycol In Water Ethylene glycol may be discharged into wastewater from its production and use. Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. Its most common use is as an automotive antifreeze. This property is exploited in some cosmetics,. Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Ethylene glycol is a clear, sweet, slightly. Ethylene Glycol In Water.
From www.researchgate.net
Single phase waterethyleneglycol heat transfer coefficient Ethylene Glycol In Water Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications. Ethylene glycol is ‘hygroscopic’ meaning it absorbs water from the atmosphere. It is obtained by the oxidation. Ethylene glycol may be discharged into wastewater from its production and use. Ethylene glycol should be avoided if there is a slightest chance of leakage to. Its most common. Ethylene Glycol In Water.
From mavink.com
Ethylene Phase Diagram Ethylene Glycol In Water Ethylene glycol should be avoided if there is a slightest chance of leakage to. This property is exploited in some cosmetics,. A 1:1 solution of ethylene glycol and. It may also enter the environment from its uses in deicing airplane runways and from spills and. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene. Ethylene Glycol In Water.
From bulkchemicals2go.com
Ethylene Glycol Wastewater Treatment Ethylene Glycol disposal Ethylene Glycol In Water Learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Its most common use is as an automotive antifreeze. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f).. Ethylene Glycol In Water.