An Automobile Cooling System Holds . The specific heat of water is 4186 j/kg. So our question says an automobile has a cooling system which holds 18 liters of water. How much heat does it. How much heat does it absorb if its. And since one kilogram is about. How much heat does it absorb if its temperature rises from 15°c to 95°c? How much heat does it absorb if its temperature rises from 15 ° c to 95 ° c ? An automobile cooling system holds 16 l of water. An automobile cooling system holds 18 l of water. An automobile cooling system holds 12 liters of water. Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature increase, it. An automobile cooling system holds 18 l of water. An automobile cooling system holds 16 l of water. The specific heat of water is 4186 j/kg⋅c∘.
from engineeringlearn.com
An automobile cooling system holds 16 l of water. An automobile cooling system holds 18 l of water. The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature increase, it. The specific heat of water is 4186 j/kg. And since one kilogram is about. The specific heat of water is 4186 j/kg⋅c∘. An automobile cooling system holds 12 liters of water. How much heat does it absorb if its. An automobile cooling system holds 18 l of water. How much heat does it absorb if its temperature rises from 15°c to 95°c?
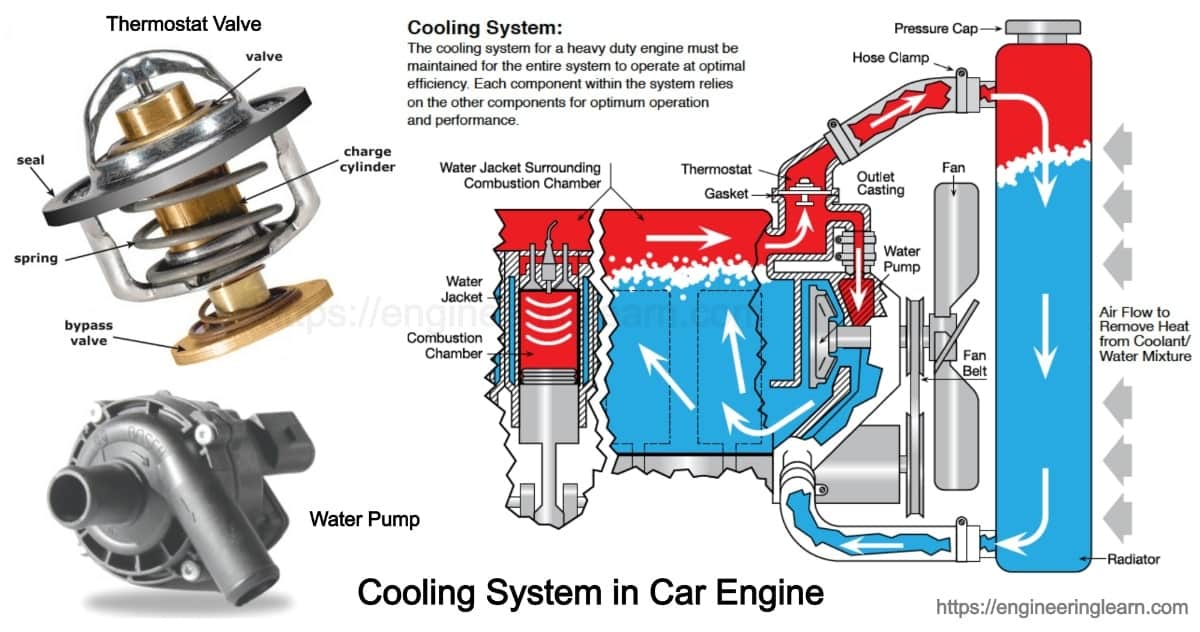
Types of Cooling System in Car Engine Components & Function
An Automobile Cooling System Holds The specific heat of water is 4186 j/kg. An automobile cooling system holds 16 l of water. An automobile cooling system holds 18 l of water. How much heat does it absorb if its temperature rises from 15°c to 95°c? So our question says an automobile has a cooling system which holds 18 liters of water. The specific heat of water is 4186 j/kg⋅c∘. An automobile cooling system holds 12 liters of water. How much heat does it absorb if its. Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. The specific heat of water is 4186 j/kg. How much heat does it absorb if its temperature rises from 15 ° c to 95 ° c ? An automobile cooling system holds 18 l of water. And since one kilogram is about. An automobile cooling system holds 16 l of water. How much heat does it. The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature increase, it.
From www.chegg.com
Solved An automobile cooling system holds 16 L of water. An Automobile Cooling System Holds The specific heat of water is 4186 j/kg⋅c∘. And since one kilogram is about. An automobile cooling system holds 18 l of water. The specific heat of water is 4186 j/kg. Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. How much heat does it. An automobile cooling system holds. An Automobile Cooling System Holds.
From www.chegg.com
Solved question 4) An automobile cooling system holds 18 L An Automobile Cooling System Holds An automobile cooling system holds 16 l of water. An automobile cooling system holds 18 l of water. The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature increase, it. How much heat does it absorb if its temperature rises from 15 ° c to 95 ° c ? How much. An Automobile Cooling System Holds.
From www.chegg.com
Solved An automobile cooling system holds 16 L of water. An Automobile Cooling System Holds An automobile cooling system holds 16 l of water. The specific heat of water is 4186 j/kg⋅c∘. How much heat does it. Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. So our question says an automobile has a cooling system which holds 18 liters of water. The task is. An Automobile Cooling System Holds.
From www.youtube.com
An automobile cooling system holds 18 L of water. How much heat does it An Automobile Cooling System Holds An automobile cooling system holds 16 l of water. How much heat does it absorb if its temperature rises from 15°c to 95°c? How much heat does it absorb if its temperature rises from 15 ° c to 95 ° c ? So our question says an automobile has a cooling system which holds 18 liters of water. How much. An Automobile Cooling System Holds.
From www.chegg.com
Solved question 4) An automobile cooling system holds 18 L An Automobile Cooling System Holds An automobile cooling system holds 16 l of water. So our question says an automobile has a cooling system which holds 18 liters of water. An automobile cooling system holds 18 l of water. An automobile cooling system holds 18 l of water. The specific heat of water is 4186 j/kg⋅c∘. An automobile cooling system holds 12 liters of water.. An Automobile Cooling System Holds.
From www.autozone.com
How Does a Car's Cooling System Work? (& How to Maintain it) An Automobile Cooling System Holds An automobile cooling system holds 16 l of water. The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature increase, it. How much heat does it. The specific heat of water is 4186 j/kg. An automobile cooling system holds 18 l of water. And since one kilogram is about. How much. An Automobile Cooling System Holds.
From www.youtube.com
Automotive cooling system, components, description, operation and more An Automobile Cooling System Holds How much heat does it absorb if its. The specific heat of water is 4186 j/kg⋅c∘. An automobile cooling system holds 16 l of water. So our question says an automobile has a cooling system which holds 18 liters of water. How much heat does it absorb if its temperature rises from 15 ° c to 95 ° c ?. An Automobile Cooling System Holds.
From medium.com
What are the different components of the car cooling system? by An Automobile Cooling System Holds And since one kilogram is about. How much heat does it absorb if its. An automobile cooling system holds 18 l of water. The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature increase, it. Show that when its temperature rises from 20 ° c to 70 ° c , it. An Automobile Cooling System Holds.
From green-way.com.ua
Глава 16 Engine cooling system Учебник по строению авто An Automobile Cooling System Holds An automobile cooling system holds 12 liters of water. And since one kilogram is about. How much heat does it. How much heat does it absorb if its temperature rises from 15°c to 95°c? The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature increase, it. How much heat does it. An Automobile Cooling System Holds.
From www.carparts.com
What's the Difference Between Your Car’s Air Conditioner & Heater? In An Automobile Cooling System Holds How much heat does it absorb if its. The specific heat of water is 4186 j/kg⋅c∘. An automobile cooling system holds 16 l of water. The specific heat of water is 4186 j/kg. The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature increase, it. How much heat does it. So. An Automobile Cooling System Holds.
From www.carparts.com
Main Components of Your Cooling System In The Garage with An Automobile Cooling System Holds Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. An automobile cooling system holds 16 l of water. How much heat does it. The specific heat of water is 4186 j/kg⋅c∘. How much heat does it absorb if its. And since one kilogram is about. An automobile cooling system holds. An Automobile Cooling System Holds.
From ceqnbuyc.blob.core.windows.net
How Does Cooling System Work In Car at Maureen Davis blog An Automobile Cooling System Holds How much heat does it absorb if its temperature rises from 15 ° c to 95 ° c ? An automobile cooling system holds 18 l of water. The specific heat of water is 4186 j/kg. Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. How much heat does it. An Automobile Cooling System Holds.
From www.chegg.com
Solved Part A An automobile cooling system holds 16 L of An Automobile Cooling System Holds An automobile cooling system holds 18 l of water. How much heat does it absorb if its temperature rises from 15°c to 95°c? An automobile cooling system holds 18 l of water. Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. An automobile cooling system holds 16 l of water.. An Automobile Cooling System Holds.
From engineeringlearn.com
Types of Cooling System in Car Engine Components & Function An Automobile Cooling System Holds How much heat does it absorb if its temperature rises from 15 ° c to 95 ° c ? An automobile cooling system holds 12 liters of water. How much heat does it. So our question says an automobile has a cooling system which holds 18 liters of water. An automobile cooling system holds 16 l of water. The task. An Automobile Cooling System Holds.
From innovationdiscoveries.space
CAR AIR CONDITIONING /AC/ SYSTEM FUNCTION COMPONENTS An Automobile Cooling System Holds An automobile cooling system holds 12 liters of water. And since one kilogram is about. The specific heat of water is 4186 j/kg. How much heat does it absorb if its temperature rises from 15 ° c to 95 ° c ? An automobile cooling system holds 16 l of water. How much heat does it absorb if its temperature. An Automobile Cooling System Holds.
From autospotrepair.com
Cooling System Repair AutoSpot Auto Repair Centerville An Automobile Cooling System Holds An automobile cooling system holds 18 l of water. An automobile cooling system holds 16 l of water. An automobile cooling system holds 12 liters of water. The specific heat of water is 4186 j/kg⋅c∘. And since one kilogram is about. An automobile cooling system holds 18 l of water. How much heat does it absorb if its temperature rises. An Automobile Cooling System Holds.
From www.chegg.com
Solved Question 6 4 pts An automobile cooling system holds 2 An Automobile Cooling System Holds And since one kilogram is about. How much heat does it absorb if its temperature rises from 15 ° c to 95 ° c ? The specific heat of water is 4186 j/kg. How much heat does it absorb if its temperature rises from 15°c to 95°c? The specific heat of water is 4186 j/kg⋅c∘. So our question says an. An Automobile Cooling System Holds.
From bgfindashop.com
How Automotive Cooling Systems Work BG Find A Shop An Automobile Cooling System Holds An automobile cooling system holds 12 liters of water. The specific heat of water is 4186 j/kg⋅c∘. An automobile cooling system holds 18 l of water. An automobile cooling system holds 16 l of water. So our question says an automobile has a cooling system which holds 18 liters of water. An automobile cooling system holds 18 l of water.. An Automobile Cooling System Holds.
From www.tescaglobal.com
Cooling System of an Automobile An Automobile Cooling System Holds An automobile cooling system holds 18 l of water. How much heat does it absorb if its temperature rises from 15 ° c to 95 ° c ? How much heat does it absorb if its. Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. How much heat does it.. An Automobile Cooling System Holds.
From www.mechanicalbooster.com
How Engine Cooling System Works? Mechanical Booster An Automobile Cooling System Holds An automobile cooling system holds 16 l of water. So our question says an automobile has a cooling system which holds 18 liters of water. How much heat does it absorb if its temperature rises from 15°c to 95°c? An automobile cooling system holds 12 liters of water. How much heat does it absorb if its temperature rises from 15. An Automobile Cooling System Holds.
From www.godigit.com
Cooling System in Automobiles Functions, Parts and Types An Automobile Cooling System Holds An automobile cooling system holds 18 l of water. Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. How much heat does it absorb if its temperature rises from 15°c to 95°c? So our question says an automobile has a cooling system which holds 18 liters of water. An automobile. An Automobile Cooling System Holds.
From manualpartdreher.z19.web.core.windows.net
Diagram Of Auto Cooling System An Automobile Cooling System Holds An automobile cooling system holds 16 l of water. An automobile cooling system holds 12 liters of water. An automobile cooling system holds 18 l of water. Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. How much heat does it absorb if its. An automobile cooling system holds 16. An Automobile Cooling System Holds.
From www.numerade.com
SOLVED points Save Answer An automobile cooling system holds 3.00 kg An Automobile Cooling System Holds An automobile cooling system holds 18 l of water. Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. The specific heat of water is 4186 j/kg⋅c∘. And since one kilogram is about. An automobile cooling system holds 18 l of water. An automobile cooling system holds 12 liters of water.. An Automobile Cooling System Holds.
From www.howacarworks.com
How an engine cooling system works How a Car Works An Automobile Cooling System Holds The specific heat of water is 4186 j/kg. An automobile cooling system holds 18 l of water. And since one kilogram is about. An automobile cooling system holds 16 l of water. The specific heat of water is 4186 j/kg⋅c∘. Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. An. An Automobile Cooling System Holds.
From engineeringdiscoveries.com
How Engine Cooling System Works? Engineering Discoveries An Automobile Cooling System Holds How much heat does it absorb if its. The specific heat of water is 4186 j/kg. The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature increase, it. An automobile cooling system holds 16 l of water. An automobile cooling system holds 18 l of water. An automobile cooling system holds. An Automobile Cooling System Holds.
From engineeringdiscoveries.com
How Engine Cooling System Works? Engineering Discoveries An Automobile Cooling System Holds The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature increase, it. The specific heat of water is 4186 j/kg⋅c∘. The specific heat of water is 4186 j/kg. And since one kilogram is about. How much heat does it absorb if its temperature rises from 15°c to 95°c? An automobile cooling. An Automobile Cooling System Holds.
From www.britannica.com
Automobile Cooling, Radiator, Engine Britannica An Automobile Cooling System Holds The specific heat of water is 4186 j/kg. How much heat does it absorb if its. An automobile cooling system holds 16 l of water. So our question says an automobile has a cooling system which holds 18 liters of water. An automobile cooling system holds 12 liters of water. An automobile cooling system holds 18 l of water. How. An Automobile Cooling System Holds.
From www.youtube.com
How a Car's Cooling System Works YouTube An Automobile Cooling System Holds Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. How much heat does it absorb if its temperature rises from 15 ° c to 95 ° c ? An automobile cooling system holds 12 liters of water. An automobile cooling system holds 16 l of water. How much heat does. An Automobile Cooling System Holds.
From dsauto.com.my
Car Cooling System What is it and How it Works? • D S Auto An Automobile Cooling System Holds And since one kilogram is about. How much heat does it. An automobile cooling system holds 12 liters of water. An automobile cooling system holds 16 l of water. The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature increase, it. An automobile cooling system holds 18 l of water. How. An Automobile Cooling System Holds.
From www.youtube.com
How an Automotive Air Conditioning System Works Explained. YouTube An Automobile Cooling System Holds An automobile cooling system holds 16 l of water. How much heat does it absorb if its. The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature increase, it. So our question says an automobile has a cooling system which holds 18 liters of water. Show that when its temperature rises. An Automobile Cooling System Holds.
From www.britannica.com
Automobile Cooling, Radiator, Engine Britannica An Automobile Cooling System Holds How much heat does it absorb if its temperature rises from 15°c to 95°c? The specific heat of water is 4186 j/kg. How much heat does it. An automobile cooling system holds 18 l of water. An automobile cooling system holds 16 l of water. An automobile cooling system holds 18 l of water. How much heat does it absorb. An Automobile Cooling System Holds.
From toytechs.com
What you need to know about your Automotive Cooling System TOYTECHS An Automobile Cooling System Holds So our question says an automobile has a cooling system which holds 18 liters of water. An automobile cooling system holds 18 l of water. An automobile cooling system holds 12 liters of water. How much heat does it absorb if its temperature rises from 15 ° c to 95 ° c ? An automobile cooling system holds 18 l. An Automobile Cooling System Holds.
From www.chegg.com
Solved An automobile cooling system holds 16 L of water. An Automobile Cooling System Holds The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature increase, it. Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. The specific heat of water is 4186 j/kg. How much heat does it absorb if its temperature rises from 15°c. An Automobile Cooling System Holds.
From www.youtube.com
How Car Cooling System Works YouTube An Automobile Cooling System Holds How much heat does it. How much heat does it absorb if its temperature rises from 15 ° c to 95 ° c ? How much heat does it absorb if its. An automobile cooling system holds 12 liters of water. An automobile cooling system holds 16 l of water. Show that when its temperature rises from 20 ° c. An Automobile Cooling System Holds.
From www.howacarworks.com
How an engine cooling system works How a Car Works An Automobile Cooling System Holds Show that when its temperature rises from 20 ° c to 70 ° c , it absorbs 60 kilocalories. An automobile cooling system holds 18 l of water. How much heat does it. The specific heat of water is 4186 j/kg. The task is to demonstrate that when an automobile cooling system, containing 12 litres of water, undergoes a temperature. An Automobile Cooling System Holds.