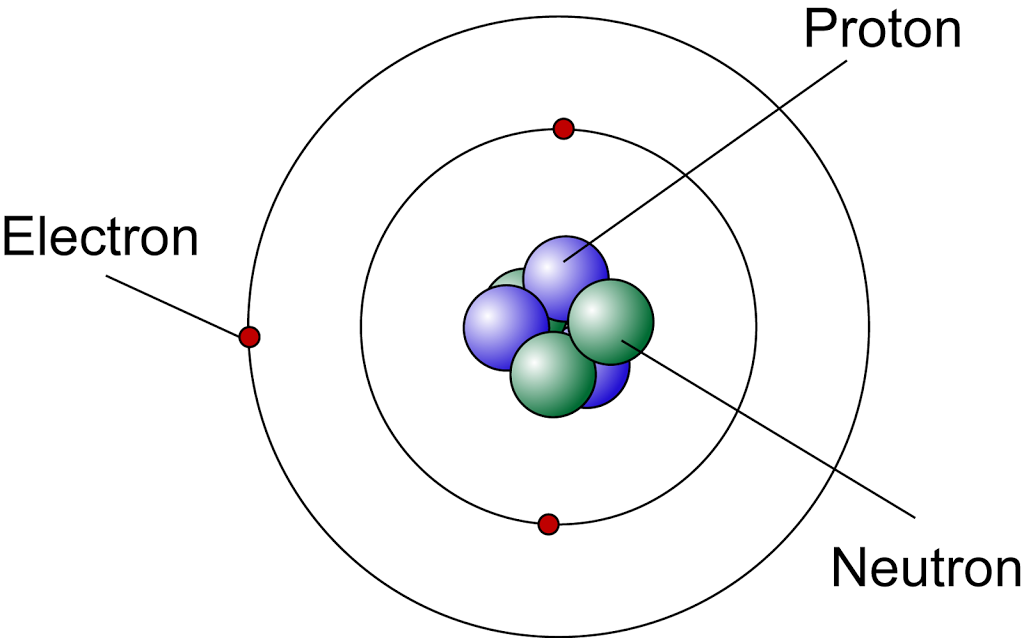
Why Do We Need Model Of The Atom . the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. this quantized atomic model, also known as the planetary model of the atom, was used to explain why the. bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect. Because the bohr model is a modification of the earlier rutherford model, some. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. niels bohr proposed the bohr model of the atom in 1915. The bohr model of the atom, a radical. learn about the bohr model of the atom.
from spmchemistry.blog.onlinetuition.com.my
niels bohr proposed the bohr model of the atom in 1915. this quantized atomic model, also known as the planetary model of the atom, was used to explain why the. The bohr model of the atom, a radical. bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. Because the bohr model is a modification of the earlier rutherford model, some. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. learn about the bohr model of the atom. niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect.
Modern Atomic Model SPM Chemistry
Why Do We Need Model Of The Atom the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. The bohr model of the atom, a radical. bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect. Because the bohr model is a modification of the earlier rutherford model, some. niels bohr proposed the bohr model of the atom in 1915. this quantized atomic model, also known as the planetary model of the atom, was used to explain why the. learn about the bohr model of the atom. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Why Do We Need Model Of The Atom niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. The bohr model of the atom,. Why Do We Need Model Of The Atom.
From sites.google.com
Unit 2 Atomic Structure Chem 20152017 Kevin Lam Why Do We Need Model Of The Atom this quantized atomic model, also known as the planetary model of the atom, was used to explain why the. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. Because the bohr model is a modification of the earlier rutherford model, some. the atomic model consists of a nucleus containing protons and neutrons, surrounded. Why Do We Need Model Of The Atom.
From www.worksheetsplanet.com
Bohr's Atomic Model Why Do We Need Model Of The Atom See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. Because the bohr model is a modification of the earlier rutherford model, some. learn about the bohr model of the atom. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. the atomic model consists. Why Do We Need Model Of The Atom.
From www.science-sparks.com
A Brief History of the Atom Why Do We Need Model Of The Atom Because the bohr model is a modification of the earlier rutherford model, some. The bohr model of the atom, a radical. bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. niels. Why Do We Need Model Of The Atom.
From classnotes.org.in
Rutherford Model of an Atom Class 9, Structure of an atom Why Do We Need Model Of The Atom See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. The bohr model of the atom, a radical. this quantized atomic model, also known as the planetary model of the atom, was used to explain why the. Because the bohr model is a modification of the earlier rutherford model, some.. Why Do We Need Model Of The Atom.
From www.hanlin.com
Edexcel A Level Physics复习笔记8.2 The Nuclear Model of the Atom翰林国际教育 Why Do We Need Model Of The Atom niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect. Because the bohr model is a modification of the earlier rutherford model, some. niels bohr proposed the bohr model of the atom in 1915. Most matter consists of an agglomeration of molecules, which can be separated relatively easily.. Why Do We Need Model Of The Atom.
From www.youtube.com
Chemistry_Class 9th_Chapter 4_Structure of the Atom_ModuleThomson's Why Do We Need Model Of The Atom See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. The bohr model of the atom, a radical. learn about the bohr model of the atom. this quantized atomic model, also known. Why Do We Need Model Of The Atom.
From www.britannica.com
Thomson atomic model Description, Plum Pudding, & Image Britannica Why Do We Need Model Of The Atom The bohr model of the atom, a radical. Because the bohr model is a modification of the earlier rutherford model, some. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. learn about the bohr model of the atom. the atomic model consists of a nucleus containing protons and. Why Do We Need Model Of The Atom.
From www.lessonplanet.com
Review Models of the Atom PPT for 10th 12th Grade Lesson Why Do We Need Model Of The Atom niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect. bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. this quantized atomic model, also known as the planetary model of the atom, was used to explain why the. Because. Why Do We Need Model Of The Atom.
From msjschemclass.blogspot.com
Ms J's Chemistry Class Atom Models and Periodic Trands Why Do We Need Model Of The Atom Because the bohr model is a modification of the earlier rutherford model, some. learn about the bohr model of the atom. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect. this quantized. Why Do We Need Model Of The Atom.
From jettgokeacevedo.blogspot.com
Describe Bohrs Model of the Atom Why Do We Need Model Of The Atom niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect. niels bohr proposed the bohr model of the atom in 1915. the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. learn about the bohr model of the atom. The. Why Do We Need Model Of The Atom.
From www.slideserve.com
PPT Bohr Model of the Atom PowerPoint Presentation, free download Why Do We Need Model Of The Atom bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. The bohr model of the atom, a radical. niels bohr proposed the bohr model of the atom in 1915. the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. Most matter consists of an. Why Do We Need Model Of The Atom.
From lah.elearningontario.ca
SNC1P Why Do We Need Model Of The Atom this quantized atomic model, also known as the planetary model of the atom, was used to explain why the. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. the atomic model consists of a. Why Do We Need Model Of The Atom.
From depositphotos.com
Atomic Models Scientific Theory Particles Physics Vector Diagram Stock Why Do We Need Model Of The Atom niels bohr proposed the bohr model of the atom in 1915. Because the bohr model is a modification of the earlier rutherford model, some. bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. learn about the bohr model of the atom. the atomic model consists of a nucleus. Why Do We Need Model Of The Atom.
From www.britannica.com
Basic concept and structure of an atom Britannica Why Do We Need Model Of The Atom niels bohr proposed the bohr model of the atom in 1915. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. Because the bohr model is a modification of the earlier rutherford model, some. the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. The bohr model of. Why Do We Need Model Of The Atom.
From mavink.com
Types Of Atomic Models Why Do We Need Model Of The Atom niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect. The bohr model of the atom, a radical. this quantized atomic model, also known as the planetary model of the atom, was used to explain why the. learn about the bohr model of the atom. bohr. Why Do We Need Model Of The Atom.
From www.compoundchem.com
The History of the Atom Theories and Models Compound Interest Why Do We Need Model Of The Atom Because the bohr model is a modification of the earlier rutherford model, some. The bohr model of the atom, a radical. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. the atomic model consists of. Why Do We Need Model Of The Atom.
From www.vecteezy.com
Atomic structure infographic as diagram for chemistry study 9016596 Why Do We Need Model Of The Atom bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. niels bohr proposed the bohr model of the atom in 1915. learn about the bohr model of the atom. niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect.. Why Do We Need Model Of The Atom.
From scienceworldfactors.blogspot.com
Concepts of atom Why Do We Need Model Of The Atom See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. Because the bohr model is a modification of the earlier rutherford model, some. niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect. The bohr model of the atom, a. Why Do We Need Model Of The Atom.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram Why Do We Need Model Of The Atom bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. Because the bohr model is a modification of the earlier rutherford model, some. learn about the bohr model of the atom. The bohr model of the atom, a radical. this quantized atomic model, also known as the planetary model of. Why Do We Need Model Of The Atom.
From mungfali.com
Atomic Structure Of An Atom Model Why Do We Need Model Of The Atom niels bohr proposed the bohr model of the atom in 1915. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. this quantized atomic model, also known as the planetary model of the atom, was used to. Why Do We Need Model Of The Atom.
From www.shalom-education.com
Bohr's Model of the Atom GCSE Physics Revision Why Do We Need Model Of The Atom bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect. The bohr model of the atom, a radical. Because the bohr model is a modification of the earlier rutherford model, some.. Why Do We Need Model Of The Atom.
From www.thinglink.com
Evolution of the Atomic Models Why Do We Need Model Of The Atom bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. The bohr model of the atom, a radical. this quantized atomic model, also known as the planetary model of the atom, was used to explain why the. the atomic model consists of a nucleus containing protons and neutrons, surrounded by. Why Do We Need Model Of The Atom.
From youreducationplus.com
IB Chemistry SL & HL 2.1 Atomic Model Why Do We Need Model Of The Atom learn about the bohr model of the atom. The bohr model of the atom, a radical. bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. niels bohr proposed the model of the. Why Do We Need Model Of The Atom.
From www.thoughtco.com
Bohr Model of the Atom Overview and Examples Why Do We Need Model Of The Atom See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. Because the bohr model is a modification of the earlier rutherford model, some. this quantized atomic model, also known as the planetary model. Why Do We Need Model Of The Atom.
From pointrelevant.blogspot.com
bohr rutherford diagram Why Do We Need Model Of The Atom See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. learn about the bohr model of the atom. The bohr model of the atom, a radical. this quantized atomic model, also known as the planetary model of the atom, was used to explain why the. bohr model, description. Why Do We Need Model Of The Atom.
From www.vecteezy.com
Different models of atom vector illustration 21669330 Vector Art at Why Do We Need Model Of The Atom bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. The bohr model of the atom, a radical. niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect. Because the bohr model is a modification of the earlier rutherford model, some.. Why Do We Need Model Of The Atom.
From www.thoughtco.com
Basic Model of the Atom Atomic Theory Why Do We Need Model Of The Atom niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. this quantized atomic model, also known as. Why Do We Need Model Of The Atom.
From spmchemistry.blog.onlinetuition.com.my
Modern Atomic Model SPM Chemistry Why Do We Need Model Of The Atom niels bohr proposed the bohr model of the atom in 1915. this quantized atomic model, also known as the planetary model of the atom, was used to explain why the. Because the bohr model is a modification of the earlier rutherford model, some. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. . Why Do We Need Model Of The Atom.
From www.youtube.com
how to make a atom model science project Bohr atomic model atomic Why Do We Need Model Of The Atom See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. The bohr model of the atom, a radical. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. niels bohr proposed the model of the atom that we still learn in school today, even though it's. Why Do We Need Model Of The Atom.
From studyrocket.co.uk
The Structure of the Atom GCSE Physics Science) AQA Why Do We Need Model Of The Atom The bohr model of the atom, a radical. See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. this quantized atomic model, also known as the planetary model of the atom, was used. Why Do We Need Model Of The Atom.
From www.expii.com
Bohr's Atomic Model — Overview & Importance Expii Why Do We Need Model Of The Atom See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. The bohr model of the atom, a radical. Because the bohr model is a modification of the earlier rutherford model, some. niels bohr proposed the bohr model of the atom in 1915. learn about the bohr model of the. Why Do We Need Model Of The Atom.
From www.youtube.com
Evolution of Atomic Model 400 BC 2020 History of the atom Timeline Why Do We Need Model Of The Atom Because the bohr model is a modification of the earlier rutherford model, some. this quantized atomic model, also known as the planetary model of the atom, was used to explain why the. niels bohr proposed the bohr model of the atom in 1915. The bohr model of the atom, a radical. bohr model, description of the structure. Why Do We Need Model Of The Atom.
From saylordotorg.github.io
The Atom Why Do We Need Model Of The Atom See the main points of the model, how to calculate absorbed or emitted energy, and why the model is. niels bohr proposed the model of the atom that we still learn in school today, even though it's technically incorrect. bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. niels. Why Do We Need Model Of The Atom.
From hubpages.com
Atoms and Atomic Structure HubPages Why Do We Need Model Of The Atom Because the bohr model is a modification of the earlier rutherford model, some. niels bohr proposed the bohr model of the atom in 1915. The bohr model of the atom, a radical. the atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. Most matter consists of an agglomeration of molecules, which can. Why Do We Need Model Of The Atom.