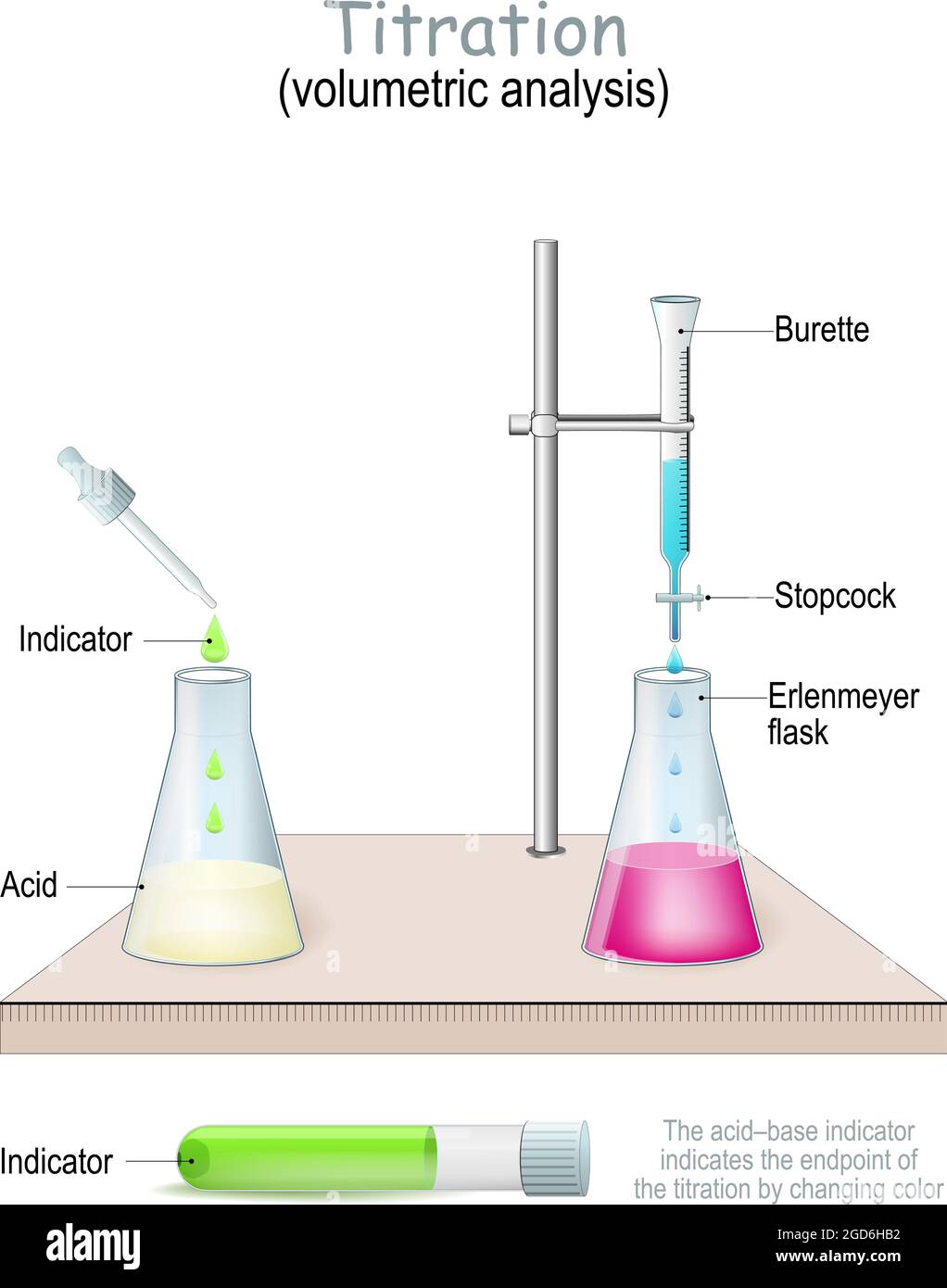
Indicator Used In Iodine Titration . Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. To know when the reaction is complete, we use starch solution as an indicator. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Solution of starch as indicator. The iodometric determination of copper in brass is both a delight and a challenge to carry out both because of the disproportionation of iodate and iodide in acid solution to produce iodine, which must be titrated quickly owing to the The starch forms a blue color when it reacts with iodine. Since aqueous iodine solutions are brown in colour, iodine can act as its own indicator. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. A platinum ring indicator electrode is used to follow the progress of the titration curve by potentiometry. However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. Iodine is freely soluble in organic solvents.
from mavink.com
It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. To know when the reaction is complete, we use starch solution as an indicator. The starch forms a blue color when it reacts with iodine. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. The iodometric determination of copper in brass is both a delight and a challenge to carry out both because of the disproportionation of iodate and iodide in acid solution to produce iodine, which must be titrated quickly owing to the Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Iodine is freely soluble in organic solvents. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. Since aqueous iodine solutions are brown in colour, iodine can act as its own indicator.
Titration Pipette
Indicator Used In Iodine Titration Iodine is freely soluble in organic solvents. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. Solution of starch as indicator. The starch forms a blue color when it reacts with iodine. The iodometric determination of copper in brass is both a delight and a challenge to carry out both because of the disproportionation of iodate and iodide in acid solution to produce iodine, which must be titrated quickly owing to the Since aqueous iodine solutions are brown in colour, iodine can act as its own indicator. Iodine is freely soluble in organic solvents. To know when the reaction is complete, we use starch solution as an indicator. A platinum ring indicator electrode is used to follow the progress of the titration curve by potentiometry. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Indicator Used In Iodine Titration Iodine is freely soluble in organic solvents. The iodometric determination of copper in brass is both a delight and a challenge to carry out both because of the disproportionation of iodate and iodide in acid solution to produce iodine, which must be titrated quickly owing to the Iodometric titration is a method used to measure the amount of an oxidizing. Indicator Used In Iodine Titration.
From exyktdgef.blob.core.windows.net
The Indicator Used In Acid Base Titration Are at Paul Guzman blog Indicator Used In Iodine Titration To know when the reaction is complete, we use starch solution as an indicator. The iodometric determination of copper in brass is both a delight and a challenge to carry out both because of the disproportionation of iodate and iodide in acid solution to produce iodine, which must be titrated quickly owing to the The starch forms a blue color. Indicator Used In Iodine Titration.
From joieldmit.blob.core.windows.net
How Is Titration Used In Medicine at Shelia Wiley blog Indicator Used In Iodine Titration Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. To know when the reaction is complete, we use starch solution as an indicator. Since aqueous iodine solutions are brown in colour, iodine can act as its own indicator. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. It works by. Indicator Used In Iodine Titration.
From analiticaderetail.com
Surichinmoi Piszkos Korlátoz ascorbic acid titration Nathaniel Ward Indicator Used In Iodine Titration Since aqueous iodine solutions are brown in colour, iodine can act as its own indicator. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. The iodometric determination of copper in brass is both a delight and a challenge to carry out both because. Indicator Used In Iodine Titration.
From www.pdfprof.com
the concentration of a solution as determined by titration meaning in hindi Indicator Used In Iodine Titration The starch forms a blue color when it reacts with iodine. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Solution of starch as indicator. Iodine is freely soluble in organic solvents. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. Thanks to its relatively low, ph independent redox potential, and reversibility. Indicator Used In Iodine Titration.
From themasterchemistry.com
Iodometric Titration Principle, Example, Advantages Indicator Used In Iodine Titration However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. A platinum ring indicator electrode is used to follow the progress of the titration curve by potentiometry. The iodometric determination of copper in brass is both a delight and a challenge to carry out both because of the disproportionation of iodate and iodide in acid. Indicator Used In Iodine Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Indicator Used In Iodine Titration However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. The iodometric determination of copper in brass is both a delight and a challenge to carry out both because of the disproportionation of iodate and iodide in acid solution to produce iodine, which must be titrated quickly owing to the Iodine exhibit a spontaneous decrease. Indicator Used In Iodine Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Indicator Used In Iodine Titration Iodine exhibit a spontaneous decrease in their concentrations by vaporization. The starch forms a blue color when it reacts with iodine. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. Iodine is freely soluble in organic solvents.. Indicator Used In Iodine Titration.
From present5.com
Dissolved Oxygen and Biochemical Oxygen Demand Analyses Prepared Indicator Used In Iodine Titration Iodine exhibit a spontaneous decrease in their concentrations by vaporization. The iodometric determination of copper in brass is both a delight and a challenge to carry out both because of the disproportionation of iodate and iodide in acid solution to produce iodine, which must be titrated quickly owing to the Iodometric titration is a method used to measure the amount. Indicator Used In Iodine Titration.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Indicator Used In Iodine Titration To know when the reaction is complete, we use starch solution as an indicator. Solution of starch as indicator. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. The iodometric determination of copper in brass is both a. Indicator Used In Iodine Titration.
From www.numerade.com
SOLVED Vitamin C (ascorbic acid, C6H8O6, 176.12 g / mol ) can be Indicator Used In Iodine Titration A platinum ring indicator electrode is used to follow the progress of the titration curve by potentiometry. Solution of starch as indicator. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. To know when the reaction is complete, we use starch solution as. Indicator Used In Iodine Titration.
From slideplayer.com
Titration Colour Changes ppt download Indicator Used In Iodine Titration However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. Iodine is freely soluble in organic solvents. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. A platinum ring indicator electrode is used to follow the progress of the titration curve by potentiometry. Solution of starch as indicator. Thanks to its relatively low, ph. Indicator Used In Iodine Titration.
From dxotuvjtw.blob.core.windows.net
What Is An Indicator In Chemistry Titration at Edward Mcraney blog Indicator Used In Iodine Titration It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Solution of starch as indicator. The iodometric determination of copper in brass is both a delight and a challenge to carry out both because of the disproportionation of iodate and iodide in acid solution to produce iodine, which must be titrated quickly owing to. Indicator Used In Iodine Titration.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Indicator Used In Iodine Titration To know when the reaction is complete, we use starch solution as an indicator. The iodometric determination of copper in brass is both a delight and a challenge to carry out both because of the disproportionation of iodate and iodide in acid solution to produce iodine, which must be titrated quickly owing to the Solution of starch as indicator. Since. Indicator Used In Iodine Titration.
From www.jlcatj.gob.mx
Iodine Solution Uses Offer Discounts, Save 66 jlcatj.gob.mx Indicator Used In Iodine Titration It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. The iodometric determination of copper in brass is both a delight and a challenge to carry out both because of the disproportionation of iodate and iodide in. Indicator Used In Iodine Titration.
From www.vernier.com
Potentiometric Titration of Aqueous Iodine Indicator Used In Iodine Titration However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. A platinum ring indicator electrode is used to follow the progress of the titration curve by potentiometry.. Indicator Used In Iodine Titration.
From studylib.net
An iodine / thiosulfate titration Indicator Used In Iodine Titration To know when the reaction is complete, we use starch solution as an indicator. Since aqueous iodine solutions are brown in colour, iodine can act as its own indicator. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. It works by mixing the oxidizing agent with iodide ions, which causes iodine to. Indicator Used In Iodine Titration.
From mungfali.com
Acid Base Titration Indicator Indicator Used In Iodine Titration To know when the reaction is complete, we use starch solution as an indicator. Solution of starch as indicator. Iodine is freely soluble in organic solvents. The starch forms a blue color when it reacts with iodine. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. It works by mixing the oxidizing agent with iodide ions, which causes iodine. Indicator Used In Iodine Titration.
From school.careers360.com
redox titration Overview, Structure, Properties & Uses Indicator Used In Iodine Titration To know when the reaction is complete, we use starch solution as an indicator. Iodine is freely soluble in organic solvents. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. The iodometric determination of copper in brass is both a delight and a. Indicator Used In Iodine Titration.
From exykwxxst.blob.core.windows.net
Finding Titration End Point at John Graves blog Indicator Used In Iodine Titration Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. A platinum ring indicator electrode is used to follow the progress of the titration curve by potentiometry. Since aqueous iodine solutions are brown in colour, iodine can act as its own indicator. The starch forms a blue color when it reacts with iodine.. Indicator Used In Iodine Titration.
From slideplayer.com
Titration Colour Changes ppt download Indicator Used In Iodine Titration Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Solution of starch as indicator. The starch forms a blue color when it reacts with iodine. However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. A platinum ring indicator electrode is used to follow the progress. Indicator Used In Iodine Titration.
From thehomeschoolscientist.com
Testing for Vitamin C with Iodine The Homeschool Scientist Indicator Used In Iodine Titration It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. Iodometric. Indicator Used In Iodine Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Indicator Used In Iodine Titration The iodometric determination of copper in brass is both a delight and a challenge to carry out both because of the disproportionation of iodate and iodide in acid solution to produce iodine, which must be titrated quickly owing to the Solution of starch as indicator. To know when the reaction is complete, we use starch solution as an indicator. Iodometric. Indicator Used In Iodine Titration.
From ceefeygo.blob.core.windows.net
Colorimetric Titration Chemistry at Michelle Church blog Indicator Used In Iodine Titration The starch forms a blue color when it reacts with iodine. To know when the reaction is complete, we use starch solution as an indicator. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. A platinum ring indicator electrode is used to follow the progress of the titration curve by potentiometry. The iodometric determination of copper in brass is. Indicator Used In Iodine Titration.
From mavink.com
Titration Pipette Indicator Used In Iodine Titration To know when the reaction is complete, we use starch solution as an indicator. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. A platinum ring indicator electrode is used to follow the progress of the titration curve by potentiometry. Since aqueous iodine solutions are brown in colour, iodine can act. Indicator Used In Iodine Titration.
From chem.libretexts.org
11.4 Reaction Stoichiometry in Solutions OxidationReduction Indicator Used In Iodine Titration However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Solution of starch as indicator. Since aqueous iodine solutions are. Indicator Used In Iodine Titration.
From www.youtube.com
Iodine / Thiosulfate Redox Titration Demonstration YouTube Indicator Used In Iodine Titration Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. The starch forms a blue color when it reacts with iodine. Since aqueous iodine solutions are brown in colour, iodine can act as its own indicator. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released.. Indicator Used In Iodine Titration.
From www.alamy.com
Phenolphthalein is used as a single indicator in acidbase titrations Indicator Used In Iodine Titration However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. The iodometric determination of copper in brass is both a delight and a challenge to carry out both because of the disproportionation of iodate and iodide in acid solution to produce iodine, which must be titrated quickly owing to the Solution of starch as indicator.. Indicator Used In Iodine Titration.
From joiyfxbtq.blob.core.windows.net
Titration Method Image at Jodie Massey blog Indicator Used In Iodine Titration Iodine exhibit a spontaneous decrease in their concentrations by vaporization. Iodine is freely soluble in organic solvents. Solution of starch as indicator. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. To know when the reaction is complete, we use starch solution as an indicator. It works by mixing the oxidizing. Indicator Used In Iodine Titration.
From www.researchgate.net
Titrating method with iodine solution Download Scientific Diagram Indicator Used In Iodine Titration Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Since aqueous iodine solutions are brown in colour, iodine can act as its own indicator. To know when the reaction is complete, we use starch solution as an indicator. Iodometric titration is a method used to measure the amount of an oxidizing. Indicator Used In Iodine Titration.
From exocowbmz.blob.core.windows.net
Indicator Used In Calcium Titration at Hattie Murphy blog Indicator Used In Iodine Titration The starch forms a blue color when it reacts with iodine. However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. To know when the reaction is complete, we use starch solution as an indicator. Iodine is freely soluble in organic solvents. A platinum ring indicator electrode is used to follow the progress of the. Indicator Used In Iodine Titration.
From mungfali.com
Acid Base Titration Indicator Indicator Used In Iodine Titration Since aqueous iodine solutions are brown in colour, iodine can act as its own indicator. The starch forms a blue color when it reacts with iodine. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Iodine. Indicator Used In Iodine Titration.
From joizlgxlm.blob.core.windows.net
Titration Experiments In Supramolecular Chemistry at Larry Ferrill blog Indicator Used In Iodine Titration Since aqueous iodine solutions are brown in colour, iodine can act as its own indicator. The starch forms a blue color when it reacts with iodine. However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. A platinum ring indicator electrode is used to follow the progress of the titration curve by potentiometry. It works. Indicator Used In Iodine Titration.
From dxonbtcto.blob.core.windows.net
Chemical Indicator AcidBase Titration at Hilda Johnston blog Indicator Used In Iodine Titration Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Iodine is freely soluble in organic solvents. However, it is quite difficult to detect endpoints using iodine colouration alone, and it is. To know when the reaction is complete, we use starch solution as an indicator. It works by mixing the oxidizing agent. Indicator Used In Iodine Titration.
From www.reddit.com
Titration using starch as an indicator for Iodine! At the end, one drop Indicator Used In Iodine Titration Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Iodine exhibit a spontaneous decrease in their concentrations by vaporization. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. The iodometric determination of copper in brass is both a delight and a challenge to carry out. Indicator Used In Iodine Titration.