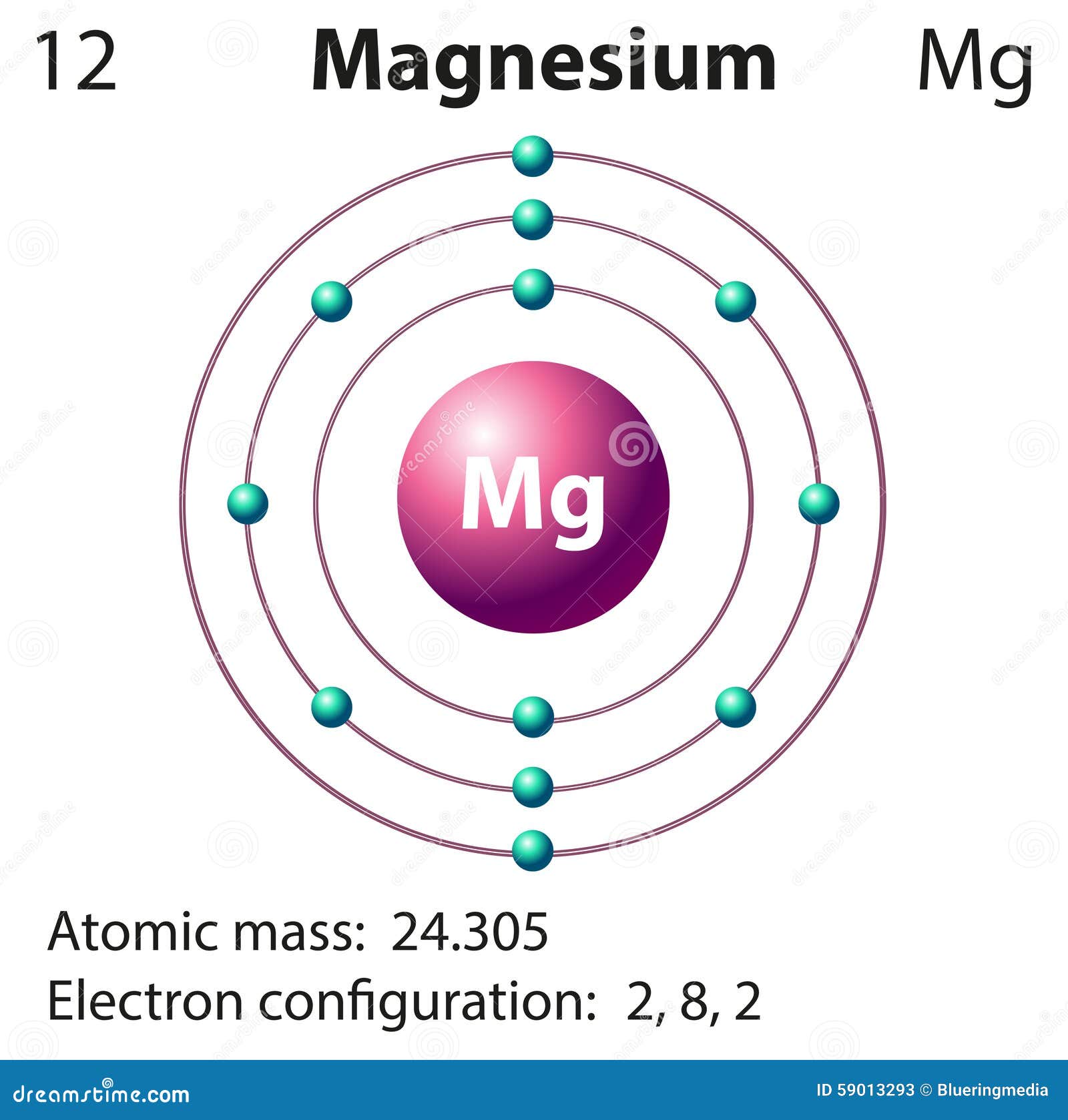
Magnesium Electron Configuration Notation . The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. Magnesium has two valence electrons in the 3s. This notation shows the distribution of electrons. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. 119 rows electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also.
from www.dreamstime.com
Magnesium has two valence electrons in the 3s. 119 rows electron configuration chart of all elements is mentioned in the table below. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. This notation shows the distribution of electrons.
Diagram Representation Of The Element Magnesium Stock Vector Image
Magnesium Electron Configuration Notation Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. 119 rows electron configuration chart of all elements is mentioned in the table below. Magnesium has two valence electrons in the 3s. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. This notation shows the distribution of electrons.
From www.britannica.com
Magnesium Description, Properties, & Compounds Britannica Magnesium Electron Configuration Notation 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. Magnesium has two valence electrons in the 3s. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. 119 rows electron configuration chart of. Magnesium Electron Configuration Notation.
From valeriadoyle.blogspot.com
orbital diagram for magnesium ValeriaDoyle Magnesium Electron Configuration Notation The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2.. Magnesium Electron Configuration Notation.
From sciencenotes.org
List of Electron Configurations of Elements Magnesium Electron Configuration Notation 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. Magnesium has two valence electrons in the 3s. 119 rows. Magnesium Electron Configuration Notation.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Electron Configuration Notation 119 rows electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. Magnesium has two valence electrons in the 3s. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. Magnesium is a chemical element with atomic number 12 which. Magnesium Electron Configuration Notation.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Electron Configuration Notation 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium is a chemical element with atomic number 12 which means there are 12 protons and. Magnesium Electron Configuration Notation.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Electron Configuration Notation 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. 119 rows electron configuration chart of all elements is mentioned in the table below.. Magnesium Electron Configuration Notation.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Configuration Notation Magnesium has two valence electrons in the 3s. This notation shows the distribution of electrons. 119 rows electron configuration chart of all elements is mentioned in the table below. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons. Magnesium Electron Configuration Notation.
From charlee-yersblogbarnes.blogspot.com
Atomic Mass of Magnesium Magnesium Electron Configuration Notation The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². This notation shows the distribution of electrons. 119 rows electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. Magnesium is a chemical element with atomic number 12 which means there are. Magnesium Electron Configuration Notation.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Configuration Notation The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. This notation shows the distribution of electrons. 119 rows electron configuration chart of all elements is mentioned in the table below. 19. Magnesium Electron Configuration Notation.
From www.youtube.com
Electronic configuration for Magnesium (Mg) spdf Trick Chemistry Magnesium Electron Configuration Notation The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. 119 rows electron configuration chart of all elements is mentioned in the table below. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus.. Magnesium Electron Configuration Notation.
From ar.inspiredpencil.com
Magnesium Electron Configuration Notation Magnesium Electron Configuration Notation Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². This notation shows the distribution of electrons. The electron configuration of magnesium. Magnesium Electron Configuration Notation.
From guidedehartsculptures.z21.web.core.windows.net
Magnesium Electric Dot Diagram Magnesium Electron Configuration Notation The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and. Magnesium Electron Configuration Notation.
From www.numerade.com
SOLVED Write the complete electron configuration for the magnesium Magnesium Electron Configuration Notation 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. Magnesium has two valence electrons in the 3s. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². This notation shows the distribution of electrons. 119. Magnesium Electron Configuration Notation.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Configuration Notation 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. This notation shows the distribution of electrons. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. Magnesium is a chemical element with atomic. Magnesium Electron Configuration Notation.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Electron Configuration Notation The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. Magnesium is a chemical. Magnesium Electron Configuration Notation.
From www.coursehero.com
[Solved] Write the short form electron configuration for magnesium Magnesium Electron Configuration Notation 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The electron configuration of. Magnesium Electron Configuration Notation.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Configuration Notation The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. This notation shows the distribution of electrons. 119 rows electron configuration chart of all elements is mentioned in the table below. Magnesium has two valence electrons in the 3s. 19 rows to determine the electron configuration of a particular atom, start at the nucleus. Magnesium Electron Configuration Notation.
From www.vectorstock.com
Symbol and electron diagram for magnesium Vector Image Magnesium Electron Configuration Notation 119 rows electron configuration chart of all elements is mentioned in the table below. Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. This notation shows the distribution of electrons. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. 19. Magnesium Electron Configuration Notation.
From www.mikrora.com
File Electron Configuration Magnesium Svg Best Diagram Collection Magnesium Electron Configuration Notation Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. This notation shows the distribution of electrons. Magnesium has two valence electrons in the 3s. 119 rows electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is 1s² 2s² 2p⁶. Magnesium Electron Configuration Notation.
From ar.inspiredpencil.com
Magnesium Electron Configuration Notation Magnesium Electron Configuration Notation The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². 119 rows electron configuration chart of all elements is mentioned in the table below. This notation shows the distribution of electrons. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. Magnesium. Magnesium Electron Configuration Notation.
From joizkfdrp.blob.core.windows.net
Magnesium Ion Aufbau Diagram at Katherine Cortez blog Magnesium Electron Configuration Notation This notation shows the distribution of electrons. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. 119 rows electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium. Magnesium Electron Configuration Notation.
From www.schoolmykids.com
Magnesium (Mg) Element Information, Facts, Properties, Uses Magnesium Electron Configuration Notation Magnesium has two valence electrons in the 3s. 119 rows electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. This notation shows the distribution of electrons. 19 rows to determine the electron configuration of a particular atom, start at the nucleus. Magnesium Electron Configuration Notation.
From www.dreamstime.com
Diagram Representation Of The Element Magnesium Stock Vector Image Magnesium Electron Configuration Notation 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The electron configuration of. Magnesium Electron Configuration Notation.
From www.animalia-life.club
Electron Dot Structure For Oxygen Magnesium Electron Configuration Notation Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s. 119 rows electron configuration. Magnesium Electron Configuration Notation.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Electron Configuration Notation The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². 119 rows electron configuration chart of all elements is mentioned in the table below. Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. Magnesium has. Magnesium Electron Configuration Notation.
From www.teachoo.com
(i) Write the electrondot structures for sodium, oxygen and magnesium Magnesium Electron Configuration Notation Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and. Magnesium Electron Configuration Notation.
From www.pinterest.co.uk
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Electron Configuration Notation Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. 119 rows electron configuration chart of all elements is mentioned in the table below. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number. Magnesium Electron Configuration Notation.
From ar.inspiredpencil.com
Magnesium Electron Configuration Notation Magnesium Electron Configuration Notation 119 rows electron configuration chart of all elements is mentioned in the table below. This notation shows the distribution of electrons. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. Magnesium has two valence electrons in the 3s. Magnesium is a. Magnesium Electron Configuration Notation.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Electron Configuration Notation This notation shows the distribution of electrons. 119 rows electron configuration chart of all elements is mentioned in the table below. Magnesium has two valence electrons in the 3s. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons. Magnesium Electron Configuration Notation.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Configuration Notation The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. This notation shows the distribution of electrons. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one. Magnesium Electron Configuration Notation.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Configuration Notation Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. Magnesium has two valence. Magnesium Electron Configuration Notation.
From ar.inspiredpencil.com
Magnesium Electron Configuration Notation Magnesium Electron Configuration Notation The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. Magnesium has two valence electrons in the 3s. 19 rows to determine the electron configuration of. Magnesium Electron Configuration Notation.
From valenceelectrons.com
Electron Configuration for Magnesium and ion(Mg2+) Magnesium Electron Configuration Notation Magnesium has two valence electrons in the 3s. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². This notation shows the distribution of electrons. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. Magnesium. Magnesium Electron Configuration Notation.
From ar.inspiredpencil.com
Electron Configuration Magnesium Magnesium Electron Configuration Notation Magnesium has two valence electrons in the 3s. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. This notation. Magnesium Electron Configuration Notation.
From exoilgobw.blob.core.windows.net
Magnesium Ion Explanation at Helen Weeks blog Magnesium Electron Configuration Notation This notation shows the distribution of electrons. 119 rows electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one. Magnesium Electron Configuration Notation.