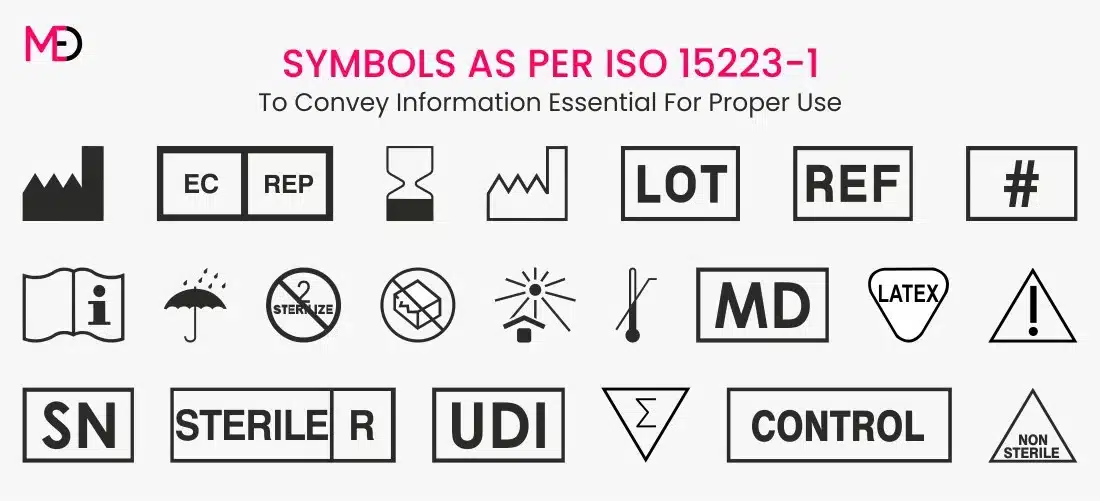
Labels Medical Device . These regulations specify the minimum requirements. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Design includes labeling content that. The general labeling requirements for medical devices are contained in 21 cfr part 801. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites.
from medicaldevicelicense.com
The general labeling requirements for medical devices are contained in 21 cfr part 801. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. Design includes labeling content that. These regulations specify the minimum requirements. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling.
Essential Medical Device Symbols for Labeling ISO 152231
Labels Medical Device Design includes labeling content that. The general labeling requirements for medical devices are contained in 21 cfr part 801. These regulations specify the minimum requirements. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. Design includes labeling content that. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites.
From mungfali.com
FDA Medical Device Label Symbols Labels Medical Device Design includes labeling content that. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. These regulations specify the minimum requirements. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. This document applies to all medical devices, including ivd medical devices, and is intended to. Labels Medical Device.
From www.flexo-graphics.com
Medical Device Labeling Archives FlexoGraphics Labels Medical Device Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. These regulations specify the. Labels Medical Device.
From clin-r.com
Labels for Medical Devices Clin R Labels Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. The general labeling requirements for medical devices are contained in 21 cfr part 801. These regulations specify the minimum requirements. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Design includes labeling content that. This. Labels Medical Device.
From templates.rjuuc.edu.np
Medical Device Label Template Labels Medical Device Design includes labeling content that. The general labeling requirements for medical devices are contained in 21 cfr part 801. These regulations specify the minimum requirements. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. This. Labels Medical Device.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Labels Medical Device This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. The general labeling requirements for medical devices are contained in 21 cfr part 801. These regulations specify the minimum requirements. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites.. Labels Medical Device.
From www.flexo-graphics.com
Medical Device Label Printing Medical Custom Labels Labels Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. These regulations specify the minimum requirements. The general labeling requirements for medical devices are contained in 21 cfr part 801. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. Design includes. Labels Medical Device.
From clin-r.com
Labels for Medical Devices Clin R Labels Medical Device This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. Design includes labeling content that. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and. Labels Medical Device.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Labels Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. The general labeling requirements for medical devices are contained in 21 cfr part 801. Design includes labeling content that. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. Principles of labelling. Labels Medical Device.
From www.unitedadlabel.com
How To Label Medical Devices Properly United Ad Label Labels Medical Device The general labeling requirements for medical devices are contained in 21 cfr part 801. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. These regulations specify the minimum requirements. Design includes labeling content that. Adequate labeling for a medical device requires proper design and procurement of the. Labels Medical Device.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Labels Medical Device Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. These regulations specify the minimum requirements. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. The general labeling requirements for medical devices are contained in 21 cfr part 801. This document applies to all medical. Labels Medical Device.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Labels Medical Device This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. These regulations specify the minimum requirements. Design includes labeling content that. The general labeling requirements for medical devices are contained in 21 cfr part 801. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb). Labels Medical Device.
From coastlabel.com
Medical Device Labeling Medical Equipment Labels Coast Label Labels Medical Device These regulations specify the minimum requirements. The general labeling requirements for medical devices are contained in 21 cfr part 801. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Design includes labeling content that. This document applies to all medical devices, including ivd medical devices, and is intended to specify the. Labels Medical Device.
From ambitiousmares.blogspot.com
34 Medical Device Label Symbols Labels Design Ideas 2020 Labels Medical Device This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. These regulations specify the. Labels Medical Device.
From data1.skinnyms.com
Medical Device Label Template Labels Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. Design includes labeling content that. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member. Labels Medical Device.
From mungfali.com
Medical Device Labeling Symbols Labels Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. These regulations specify the minimum requirements. Design includes labeling content that. This document applies to all medical devices, including ivd medical devices, and is intended to. Labels Medical Device.
From barcode-labels.com
Medical Device Labels Electronic Imaging Materials Labels Medical Device Design includes labeling content that. These regulations specify the minimum requirements. The general labeling requirements for medical devices are contained in 21 cfr part 801. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. This. Labels Medical Device.
From labelbasic.com
Using Medical Device Labels Without Complications Labels Medical Device The general labeling requirements for medical devices are contained in 21 cfr part 801. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. These regulations specify the minimum requirements. Design includes. Labels Medical Device.
From mungfali.com
Medical Device Labeling Symbols Labels Medical Device These regulations specify the minimum requirements. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb). Labels Medical Device.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Labels Medical Device The general labeling requirements for medical devices are contained in 21 cfr part 801. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. These regulations specify the minimum requirements. Design includes labeling content that. Adequate labeling for a medical device requires proper design and procurement of the. Labels Medical Device.
From www.directindustry.com
Identification label Medical Device Labels CILS International Labels Medical Device Design includes labeling content that. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. The general labeling requirements for medical devices are contained in 21 cfr part 801. These regulations specify the minimum requirements. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. This. Labels Medical Device.
From www.afpharmaservice.com
Medical Device Labelling Requirements Labels Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. These regulations specify the minimum requirements. Design includes labeling content that. This document applies to all medical devices, including ivd medical devices, and is intended to. Labels Medical Device.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Labels Medical Device Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. The general labeling requirements for medical devices are contained in 21 cfr part 801. Design includes labeling content that. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. These. Labels Medical Device.
From www.tailoredlabel.com
UDI Label Requirements For FDA Medical Device Labels TLP Labels Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. These regulations specify the minimum requirements. Design includes labeling content that. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. Principles of labelling for medical devices and ivd medical devices pdf. Labels Medical Device.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Labels Medical Device The general labeling requirements for medical devices are contained in 21 cfr part 801. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. These regulations specify the minimum requirements. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. This document applies to all medical. Labels Medical Device.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Labels Medical Device Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Design includes labeling content that. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. Adequate labeling for a medical device requires proper design and procurement of the labels and. Labels Medical Device.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Labels Medical Device The general labeling requirements for medical devices are contained in 21 cfr part 801. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. These regulations specify the minimum requirements. Design includes. Labels Medical Device.
From mavink.com
Medical Device Labeling Symbols Labels Medical Device These regulations specify the minimum requirements. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Design includes labeling content that. The general labeling requirements for medical devices are contained in 21 cfr part 801. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. This. Labels Medical Device.
From www.tapecon.com
What Information Should You Include on Your Medical Device Label? Labels Medical Device The general labeling requirements for medical devices are contained in 21 cfr part 801. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. These regulations specify the minimum requirements. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Design includes labeling content that. This. Labels Medical Device.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Labels Medical Device Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. Design includes labeling content that. These regulations specify the minimum requirements. The general labeling requirements for medical devices are contained. Labels Medical Device.
From www.freseniusmedicalcare.com
MedizinprodukteVerordnung Fresenius Medical Care Labels Medical Device These regulations specify the minimum requirements. The general labeling requirements for medical devices are contained in 21 cfr part 801. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of.. Labels Medical Device.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Labels Medical Device Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. The general labeling requirements for medical devices are contained in 21 cfr part 801. Design includes labeling content that. These regulations specify the minimum requirements. This. Labels Medical Device.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Labels Medical Device The general labeling requirements for medical devices are contained in 21 cfr part 801. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format of. These regulations specify the minimum requirements. Design includes labeling content that. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb). Labels Medical Device.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Labels Medical Device Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Design includes labeling content that. This document applies to all medical devices, including ivd medical devices, and is intended to specify the general content and format. Labels Medical Device.
From www.resourcelabel.com
Traceable Medical Device Labeling Resource Label Group Labels Medical Device The general labeling requirements for medical devices are contained in 21 cfr part 801. Design includes labeling content that. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. These regulations specify the minimum requirements. This. Labels Medical Device.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Labels Medical Device The general labeling requirements for medical devices are contained in 21 cfr part 801. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Design includes labeling content that. This document applies to all medical devices,. Labels Medical Device.