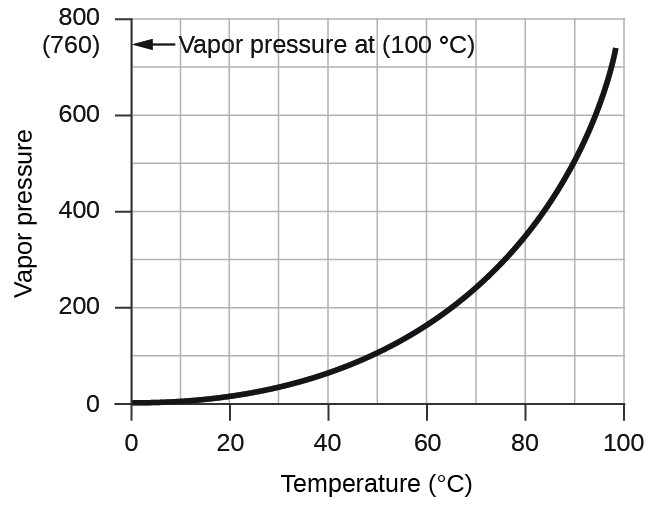
What Is Vapour-Pressure Curve . It is important to note that when a. A vapor pressure curve is a graph of vapor pressure as a function of temperature. A vapor pressure curve is a graph of vapor pressure as a function of temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. To know how and why the vapor pressure of a liquid varies with temperature. To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. To find the normal boiling point of liquid, a horizontal line is.
from pressbooks.online.ucf.edu
That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: A vapor pressure curve is a graph of vapor pressure as a function of temperature. To know how and why the vapor pressure of a liquid varies with temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); A vapor pressure curve is a graph of vapor pressure as a function of temperature. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. It is important to note that when a. To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. To find the normal boiling point of liquid, a horizontal line is.
9.4 Mixtures of Gases and Partial Pressures Chemistry Fundamentals
What Is Vapour-Pressure Curve A vapor pressure curve is a graph of vapor pressure as a function of temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. To find the normal boiling point of liquid, a horizontal line is. To know how and why the vapor pressure of a liquid varies with temperature. It is important to note that when a. A vapor pressure curve is a graph of vapor pressure as a function of temperature. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. A vapor pressure curve is a graph of vapor pressure as a function of temperature.
From www.physicsforums.com
Saturated vapour pressure vapour quality and TS diagram location What Is Vapour-Pressure Curve Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: To know how and why the vapor pressure of a liquid varies with temperature. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. To find the normal boiling point of a liquid, a horizontal line. What Is Vapour-Pressure Curve.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel What Is Vapour-Pressure Curve The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); To know how and why the vapor pressure of a liquid varies with temperature. To find the normal boiling point of liquid, a horizontal line is. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the.. What Is Vapour-Pressure Curve.
From www.numerade.com
SOLVED Over a water surface, the saturation vapor pressure (es) is What Is Vapour-Pressure Curve The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. To find the normal boiling point of liquid, a horizontal line is. A vapor pressure curve is a graph of vapor pressure as a function of. What Is Vapour-Pressure Curve.
From essayparlour.com
Question Consider the vapor pressure curves for H2O and CCl4 study Bay What Is Vapour-Pressure Curve To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by. What Is Vapour-Pressure Curve.
From vacaero.com
VapourPressureCurvesforVariousMaterials Vacaero What Is Vapour-Pressure Curve A vapor pressure curve is a graph of vapor pressure as a function of temperature. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. The vapor pressure of a liquid is the equilibrium pressure of a vapor. What Is Vapour-Pressure Curve.
From www.researchgate.net
Vapour pressure curve (solid line), critical point (black circle) and What Is Vapour-Pressure Curve It is important to note that when a. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); To know how and why the vapor pressure of a liquid varies with temperature. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. A vapor. What Is Vapour-Pressure Curve.
From brainly.in
Draw vapour pressure composition diagram and corresponding boiling What Is Vapour-Pressure Curve To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. A vapor pressure curve is a graph of vapor pressure as a function of temperature. To know how and why the vapor pressure of a liquid varies with temperature. The vapor pressure of a liquid is the equilibrium pressure of. What Is Vapour-Pressure Curve.
From pressbooks.bccampus.ca
2.3 Phase diagrams Introduction to Engineering Thermodynamics What Is Vapour-Pressure Curve To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. To know how and why the vapor pressure of a liquid varies with temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapour pressure, also known as vapour equilibrium pressure,. What Is Vapour-Pressure Curve.
From www.nuclear-power.com
Saturation Vapor Curve Nuclear Power What Is Vapour-Pressure Curve To know how and why the vapor pressure of a liquid varies with temperature. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. To find the normal boiling. What Is Vapour-Pressure Curve.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is Vapour-Pressure Curve To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. To know how and why the vapor pressure of a liquid varies with temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); A vapor pressure curve is a graph of. What Is Vapour-Pressure Curve.
From www.powerstream.com
Vapor pressures of the Chemical Elements, vapor pressure of metals and What Is Vapour-Pressure Curve Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. To find the normal boiling point of liquid, a horizontal. What Is Vapour-Pressure Curve.
From www.slideserve.com
PPT PHASE SEPARATIONS PowerPoint Presentation, free download ID1996100 What Is Vapour-Pressure Curve It is important to note that when a. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. To find the normal boiling point of liquid, a horizontal line is. To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. The vapor pressure of. What Is Vapour-Pressure Curve.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID1025977 What Is Vapour-Pressure Curve Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. It is important to note that when a. That is, the pressure of the vapor resulting from evaporation of. What Is Vapour-Pressure Curve.
From www.researchgate.net
The effect of temperature on saturation vapor pressure, actual vapor What Is Vapour-Pressure Curve To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. A. What Is Vapour-Pressure Curve.
From www.engineeringtoolbox.com
Hydrocarbones Vapor Pressure What Is Vapour-Pressure Curve A vapor pressure curve is a graph of vapor pressure as a function of temperature. It is important to note that when a. To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. To know how and why the vapor pressure of a liquid varies with temperature. To understand that. What Is Vapour-Pressure Curve.
From www.doubtnut.com
The vapour pressure curves of the same solute in the same solvent are What Is Vapour-Pressure Curve To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. A vapor pressure curve is a graph of vapor pressure as a function of temperature. Vapour pressure, also known as vapour equilibrium pressure, can. What Is Vapour-Pressure Curve.
From www.numerade.com
SOLVEDUse the vapor pressure curves shown in the figure below for What Is Vapour-Pressure Curve The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); A vapor pressure curve is a graph of vapor pressure as a function of temperature. To know how and why the vapor pressure of a liquid varies with temperature. To understand that the equilibrium vapor pressure of a liquid depends on the. What Is Vapour-Pressure Curve.
From saylordotorg.github.io
Vapor Pressure What Is Vapour-Pressure Curve To know how and why the vapor pressure of a liquid varies with temperature. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. A. What Is Vapour-Pressure Curve.
From classnotes.org.in
Vapour Pressure Chemistry, Class 11, States of Matter What Is Vapour-Pressure Curve Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. A vapor pressure curve is a graph of vapor pressure as a function of temperature. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressures. What Is Vapour-Pressure Curve.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download What Is Vapour-Pressure Curve To find the normal boiling point of liquid, a horizontal line is. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. The vapor pressure of a liquid is. What Is Vapour-Pressure Curve.
From www.researchgate.net
The saturation curve of pressure against temperature for chlorine What Is Vapour-Pressure Curve A vapor pressure curve is a graph of vapor pressure as a function of temperature. To find the normal boiling point of liquid, a horizontal line is. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. A vapor pressure curve is a graph of. What Is Vapour-Pressure Curve.
From www.engineeringtoolbox.com
Water Saturation Pressure vs. Temperature What Is Vapour-Pressure Curve It is important to note that when a. To know how and why the vapor pressure of a liquid varies with temperature. To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. The vapor. What Is Vapour-Pressure Curve.
From www.researchgate.net
Vapour pressure curve for gasoline, with lower and upper flammability What Is Vapour-Pressure Curve A vapor pressure curve is a graph of vapor pressure as a function of temperature. A vapor pressure curve is a graph of vapor pressure as a function of temperature. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: It is important to note that when a. That is, the pressure of the. What Is Vapour-Pressure Curve.
From www.chegg.com
Solved 18. Vapour pressure curves for substances (A) and (B) What Is Vapour-Pressure Curve That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. A vapor pressure curve is a graph of vapor pressure as a function of temperature. To find the normal boiling point of liquid, a horizontal line is. A vapor pressure curve is a graph of vapor pressure as a function of temperature.. What Is Vapour-Pressure Curve.
From chart-studio.plotly.com
Vapor Pressure Curves for Propane and Water line chart made by What Is Vapour-Pressure Curve That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. A vapor pressure curve is a graph of vapor pressure as a function of temperature. To know how and why the vapor pressure of a liquid varies with temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor. What Is Vapour-Pressure Curve.
From socratic.org
Explain how each of the following affects the vapour pressure of a What Is Vapour-Pressure Curve Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. To find the normal boiling point of liquid, a horizontal line is. A vapor pressure curve is a graph of vapor pressure as a function of temperature. That is, the pressure of the vapor resulting. What Is Vapour-Pressure Curve.
From www.chegg.com
Solved Use the vapour pressure curves illustrated here to What Is Vapour-Pressure Curve That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); A vapor pressure curve is a graph of. What Is Vapour-Pressure Curve.
From saylordotorg.github.io
Properties of Liquids What Is Vapour-Pressure Curve To know how and why the vapor pressure of a liquid varies with temperature. A vapor pressure curve is a graph of vapor pressure as a function of temperature. A vapor pressure curve is a graph of vapor pressure as a function of temperature. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the.. What Is Vapour-Pressure Curve.
From www.boiler-planning.com
Boiling pressure and temperature Bosch Steam boiler planning What Is Vapour-Pressure Curve It is important to note that when a. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. A vapor pressure curve is a graph of vapor pressure as a function of temperature. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: To find the. What Is Vapour-Pressure Curve.
From quizizz.com
Vapor Pressure and Intermolecular forces Quizizz What Is Vapour-Pressure Curve To find the normal boiling point of liquid, a horizontal line is. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. To know how and why the vapor pressure of a liquid varies with temperature.. What Is Vapour-Pressure Curve.
From www.researchgate.net
Vapourliquid equilibrium of ethanolwater showing distillation steps What Is Vapour-Pressure Curve To find the normal boiling point of liquid, a horizontal line is. To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: To understand that the equilibrium vapor pressure of a liquid depends on. What Is Vapour-Pressure Curve.
From pressbooks.online.ucf.edu
9.4 Mixtures of Gases and Partial Pressures Chemistry Fundamentals What Is Vapour-Pressure Curve Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. A vapor pressure curve is a graph of vapor pressure as a function of temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); To find. What Is Vapour-Pressure Curve.
From kimray.com
What's the Difference between Hydrocarbon Dew Point and Water Vapor Dew What Is Vapour-Pressure Curve To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: To find the normal boiling point of liquid, a horizontal line is. Vapour pressure, also known as vapour equilibrium pressure, can be defined as. What Is Vapour-Pressure Curve.
From www.chegg.com
Solved Shown below is the vapor pressure curve for water What Is Vapour-Pressure Curve To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure. To know how and why the vapor pressure of a liquid varies with temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapour pressure, also known as vapour equilibrium pressure,. What Is Vapour-Pressure Curve.
From askfilo.com
Q.84 The vapour pressure vs, temperature curve for a solution solvent sys.. What Is Vapour-Pressure Curve To know how and why the vapor pressure of a liquid varies with temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the. Vapour pressure, also known as vapour equilibrium pressure, can be defined as. What Is Vapour-Pressure Curve.