Lab Report Calorimetry . One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. The first part of the lab session is devoted to evaluating ccal of your calorimeter. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. The solutions that you are studying should be placed in the in‐. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Calorimetry is used to measure amounts of heat transferred to. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Enthalpy of combustion of a metal You use an aluminum vessel as a calorimeter. Lab session 9, experiment 8: Metal oxide + hcl reaction;
from www.studocu.com
Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. The solutions that you are studying should be placed in the in‐. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Calorimetry is used to measure amounts of heat transferred to. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. You use an aluminum vessel as a calorimeter. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Enthalpy of combustion of a metal The first part of the lab session is devoted to evaluating ccal of your calorimeter. Lab session 9, experiment 8:
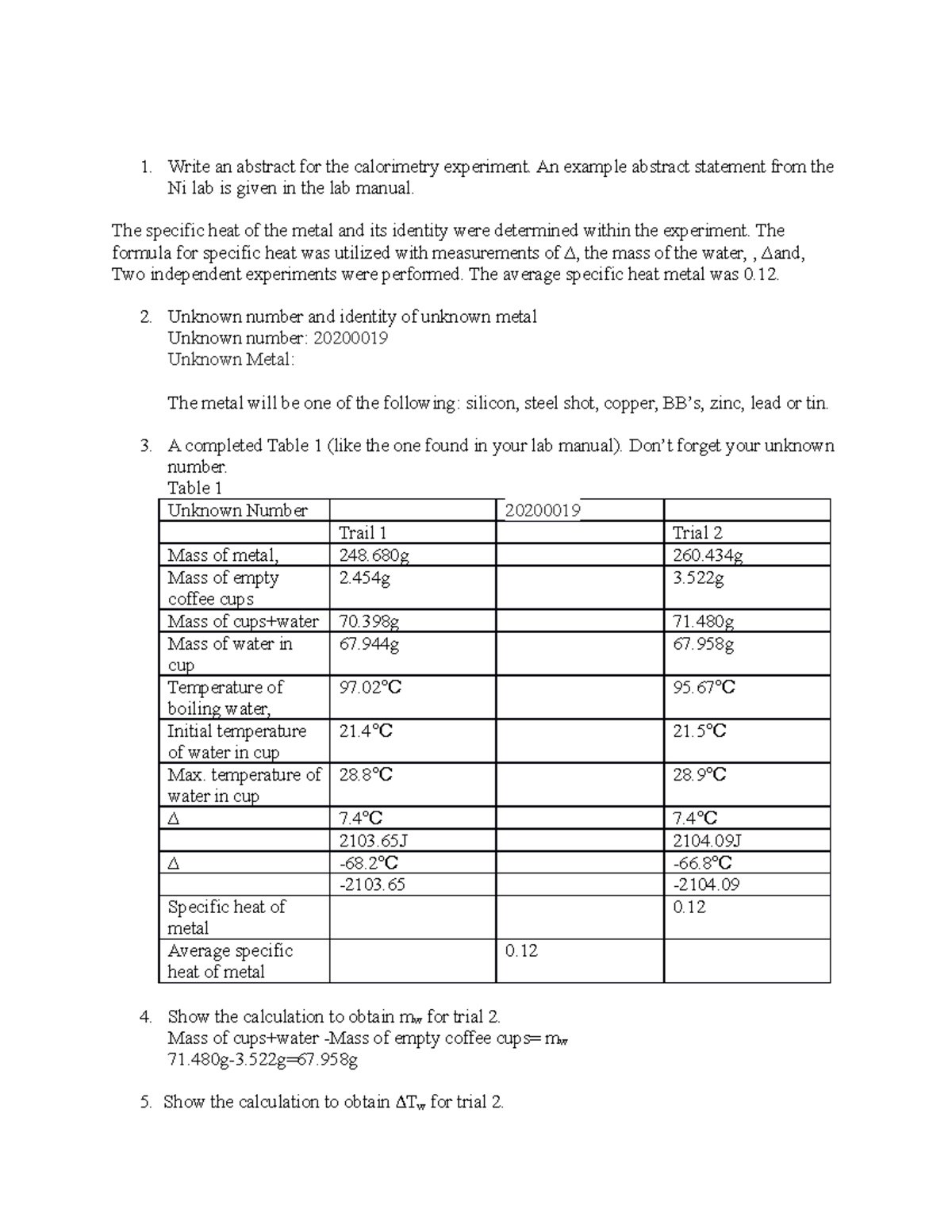
Calorimetry Lab Report Write an abstract for the calorimetry
Lab Report Calorimetry Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. You use an aluminum vessel as a calorimeter. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Enthalpy of combustion of a metal The first part of the lab session is devoted to evaluating ccal of your calorimeter. Calorimetry is used to measure amounts of heat transferred to. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Lab session 9, experiment 8: The solutions that you are studying should be placed in the in‐. Metal oxide + hcl reaction; Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Lab Report Calorimetry One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Lab session 9, experiment 8: Enthalpy of combustion of a metal Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. The solutions that you are studying should. Lab Report Calorimetry.
From www.studocu.com
Lab ReportCalorimetry Lab Report Calorimetry Objective Safety Lab Report Calorimetry In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. One technique we can use to measure the amount. Lab Report Calorimetry.
From studylib.net
Calorimetry Lab Specific Heat Capacity Lab Report Calorimetry Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. The solutions that you are studying should be placed in the in‐. The first part of the lab session is devoted to evaluating ccal of your calorimeter. One technique we can use to measure the amount of heat involved in. Lab Report Calorimetry.
From www.pdfprof.com
calorimetry experiment lab report conclusion Lab Report Calorimetry Enthalpy of combustion of a metal The first part of the lab session is devoted to evaluating ccal of your calorimeter. Metal oxide + hcl reaction; Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. You use. Lab Report Calorimetry.
From www.studocu.com
Calorimetry Lab Report Experiment 6. Calorimetry Purpose The purpose Lab Report Calorimetry Lab session 9, experiment 8: One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Calorimetry is used to measure amounts of heat transferred to. Calorimetry, heat of. Lab Report Calorimetry.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Lab Report Calorimetry You use an aluminum vessel as a calorimeter. Enthalpy of combustion of a metal Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Lab session 9, experiment 8: Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using. Lab Report Calorimetry.
From www.studocu.com
Calorimetry Lab Report Report Calorimetry Part A Data Sheet Report Lab Report Calorimetry Lab session 9, experiment 8: Metal oxide + hcl reaction; One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The first part of the lab session is devoted to evaluating ccal of your calorimeter. Calorimetry is the study of heat transferred in a chemical reaction, and a. Lab Report Calorimetry.
From studylib.net
Calorimetry Lab Report Problem To experimentally determine the Lab Report Calorimetry Enthalpy of combustion of a metal Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Calorimetry is used to measure amounts of heat. Lab Report Calorimetry.
From studylib.net
Student instructions and lab report Lab Report Calorimetry Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Lab session 9, experiment 8: Enthalpy of combustion of a metal The first part of the lab session is devoted to evaluating ccal of your calorimeter. Calorimetry is used to measure amounts of heat transferred to. Metal oxide. Lab Report Calorimetry.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Lab Report Calorimetry Lab session 9, experiment 8: You use an aluminum vessel as a calorimeter. Metal oxide + hcl reaction; Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Calorimetry is used to measure amounts of heat transferred to. Enthalpy of combustion of a metal One technique we can. Lab Report Calorimetry.
From www.chegg.com
Solved Experiment 25 Report Sheet Calorimetry Date Lab Sec Lab Report Calorimetry Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. The solutions that you are studying should be placed in the in‐. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Metal oxide + hcl. Lab Report Calorimetry.
From answerhappy.com
CALORIMETRY HEAT CAPACITY OF A CALORIMETER NTRODUCTION Lab Data Lab Report Calorimetry Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Metal oxide + hcl reaction; In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. The first part of the lab. Lab Report Calorimetry.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Lab Report Calorimetry The first part of the lab session is devoted to evaluating ccal of your calorimeter. Enthalpy of combustion of a metal Lab session 9, experiment 8: Metal oxide + hcl reaction; In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. You use. Lab Report Calorimetry.
From www.chegg.com
Solved Calorimetry PostLab Report (a) Report the collected Lab Report Calorimetry In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. The solutions that you are studying should be placed in the in‐. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Calorimetry. Lab Report Calorimetry.
From www.studocu.com
Calorimetry Lab Report Name Mahma Ahmed Partner Wala Naeem Student Lab Report Calorimetry Enthalpy of combustion of a metal In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. You use an aluminum vessel as a calorimeter. The solutions that you are studying should be placed in the in‐. Calorimetry, heat of reaction specific heat is. Lab Report Calorimetry.
From www.studocu.com
Calorimetry Lab Lab report. Experiment 6. (1 week) (LCA Lab Report Calorimetry Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Metal oxide + hcl reaction; You use an aluminum vessel as a calorimeter. Calorimetry is used to measure amounts of heat transferred to. In this experiment you will heat a known mass of a metal to a known temperature and. Lab Report Calorimetry.
From www.studocu.com
Formal Lab Report Calorimetry Experiment 25 CALORIMETRY Abstract Lab Report Calorimetry Lab session 9, experiment 8: One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Enthalpy of combustion of a metal Calorimetry is used to measure amounts of heat transferred to. Metal oxide + hcl reaction; In this experiment you will heat a known mass of a metal. Lab Report Calorimetry.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Lab Report Calorimetry Enthalpy of combustion of a metal Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. The first part of the lab session is devoted to evaluating ccal of your calorimeter. One technique we can use to measure the amount of heat involved in a chemical. Lab Report Calorimetry.
From www.scribd.com
Pre Lab Report Calorimetry PDF Lab Report Calorimetry You use an aluminum vessel as a calorimeter. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Metal oxide + hcl reaction; Lab session 9, experiment 8: The solutions that you are studying should be placed in the in‐. The first part of the lab session is. Lab Report Calorimetry.
From studylib.net
06.03 Calorimetry Lab Report Lab Report Calorimetry In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. The solutions that you are studying should be placed in the in‐. The first part of the lab session is devoted to evaluating ccal of your calorimeter. Calorimetry, heat of reaction specific heat. Lab Report Calorimetry.
From www.studocu.com
EXP 25 Calorimetry Chiara De Jesus Lab Partners Niah J., Harshita Lab Report Calorimetry Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. The solutions that you are studying should be placed in the in‐. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Enthalpy of combustion of. Lab Report Calorimetry.
From www.chegg.com
Solved Experiment 25 Report Sheet Calorimetry Lab Sec Name Lab Report Calorimetry Metal oxide + hcl reaction; Calorimetry is used to measure amounts of heat transferred to. Enthalpy of combustion of a metal The solutions that you are studying should be placed in the in‐. You use an aluminum vessel as a calorimeter. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using. Lab Report Calorimetry.
From www.studocu.com
Calorimetry LAB Report Calorimetry Enthalpy of Neutralization Lab Lab Report Calorimetry Metal oxide + hcl reaction; Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Calorimetry is used to measure amounts of heat transferred to. Lab session 9, experiment 8: In this experiment you will heat a known mass of a metal to a known temperature. Lab Report Calorimetry.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Lab Report Calorimetry Calorimetry is used to measure amounts of heat transferred to. You use an aluminum vessel as a calorimeter. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Metal oxide + hcl reaction; Abstract the main purpose of the calorimetry experiment is to. Lab Report Calorimetry.
From www.studocu.com
Calorimetry Lab Chem 112 General Chemistry StuDocu Lab Report Calorimetry Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this experiment you will heat a known mass of a metal to a known temperature and then. Lab Report Calorimetry.
From www.youtube.com
How to calculate analysis results L3 Lab Report Calorimetry YouTube Lab Report Calorimetry In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Enthalpy of combustion of a metal Lab session 9, experiment 8: You use an aluminum vessel as a calorimeter. One technique we can use to measure the amount of heat involved in a. Lab Report Calorimetry.
From www.studocu.com
Lab Report Calorimetry Lab Report CALORIMETRY Introduction The Lab Report Calorimetry Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Lab session 9, experiment 8: One technique we can use to. Lab Report Calorimetry.
From www.studocu.com
P calorimetry 25 lab report StuDocu Lab Report Calorimetry Enthalpy of combustion of a metal The first part of the lab session is devoted to evaluating ccal of your calorimeter. Metal oxide + hcl reaction; One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Lab session 9, experiment 8: The solutions that you are studying should. Lab Report Calorimetry.
From www.thinkswap.com
Calorimetry Lab Report PHYS1006 Foundations of Physics Curtin Lab Report Calorimetry Enthalpy of combustion of a metal Lab session 9, experiment 8: The first part of the lab session is devoted to evaluating ccal of your calorimeter. Metal oxide + hcl reaction; The solutions that you are studying should be placed in the in‐. In this experiment you will heat a known mass of a metal to a known temperature and. Lab Report Calorimetry.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Lab Report Calorimetry In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Metal oxide + hcl reaction; Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. The first part of the lab session is. Lab Report Calorimetry.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Lab Report Calorimetry The solutions that you are studying should be placed in the in‐. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Lab session 9, experiment 8: The. Lab Report Calorimetry.
From www.scribd.com
Lab 4 Calorimetry Lab PDF Temperature Heat Capacity Lab Report Calorimetry Lab session 9, experiment 8: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. You use an aluminum. Lab Report Calorimetry.
From www.chegg.com
Solved Lab 5 Report Calorimetry Determination of Specific Lab Report Calorimetry Calorimetry is used to measure amounts of heat transferred to. The solutions that you are studying should be placed in the in‐. Metal oxide + hcl reaction; Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Lab session 9, experiment 8: Abstract the main purpose of the. Lab Report Calorimetry.
From www.studocu.com
Caloremetry final Calorimetry Lab Report Calorimetry Objectives Lab Report Calorimetry The solutions that you are studying should be placed in the in‐. You use an aluminum vessel as a calorimeter. The first part of the lab session is devoted to evaluating ccal of your calorimeter. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains. Lab Report Calorimetry.
From www.chegg.com
Solved LAB REPORT CALORIMETRY Table 11.1 Specific heat. Lab Report Calorimetry Calorimetry is used to measure amounts of heat transferred to. Lab session 9, experiment 8: One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The solutions that you are studying should be placed in the in‐. You use an aluminum vessel as a calorimeter. Calorimetry is the. Lab Report Calorimetry.