Table Salt Covalent Bond . Table sugar or sucrose differs from salt in the bonding between its atoms. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: In salt, the sodium atom donates its electron, so it yields the na + ion in water, while the. This type of bonding occurs between two atoms of the same. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a stable ionic. Covalent bonding is the sharing of electrons between atoms. Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1 ratio of sodium. Nevertheless, the compound has properties completely different from either elemental sodium. Examples of compounds with ionic bonds include salt, such as table salt (nacl). The atoms in sugar do not form ions; Table salt, as we have seen, consists of only two elements:
from www.slideserve.com
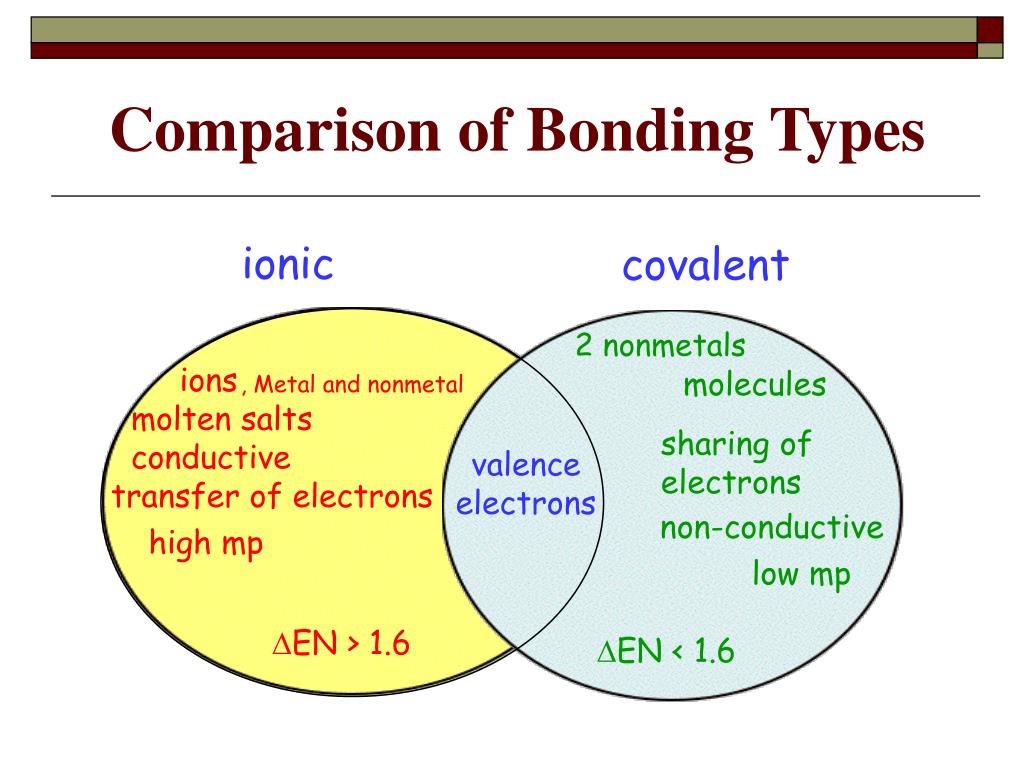
Covalent bonding is the sharing of electrons between atoms. This type of bonding occurs between two atoms of the same. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a stable ionic. Table sugar or sucrose differs from salt in the bonding between its atoms. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: The atoms in sugar do not form ions; In salt, the sodium atom donates its electron, so it yields the na + ion in water, while the. Nevertheless, the compound has properties completely different from either elemental sodium. Examples of compounds with ionic bonds include salt, such as table salt (nacl). Table salt, as we have seen, consists of only two elements:
PPT Chemical Bonds PowerPoint Presentation, free download ID6126176
Table Salt Covalent Bond In salt, the sodium atom donates its electron, so it yields the na + ion in water, while the. Covalent bonding is the sharing of electrons between atoms. Examples of compounds with ionic bonds include salt, such as table salt (nacl). Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Table salt, as we have seen, consists of only two elements: The atoms in sugar do not form ions; This type of bonding occurs between two atoms of the same. In salt, the sodium atom donates its electron, so it yields the na + ion in water, while the. Table sugar or sucrose differs from salt in the bonding between its atoms. Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1 ratio of sodium. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a stable ionic. Nevertheless, the compound has properties completely different from either elemental sodium.
From www.numerade.com
SOLVED Covalent bonds occur when ions of opposite charge are attracted Table Salt Covalent Bond In salt, the sodium atom donates its electron, so it yields the na + ion in water, while the. Table sugar or sucrose differs from salt in the bonding between its atoms. Examples of compounds with ionic bonds include salt, such as table salt (nacl). An atom of sodium (na) donates one of its electrons to an atom of chlorine. Table Salt Covalent Bond.
From bricejurban.github.io
The chemical formula for an Ionic Compound is the simplest electrically Table Salt Covalent Bond Table salt, as we have seen, consists of only two elements: Examples of compounds with ionic bonds include salt, such as table salt (nacl). Covalent bonding is the sharing of electrons between atoms. Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1 ratio of sodium. The atoms in sugar do. Table Salt Covalent Bond.
From sciencenotes.org
Examples of Ionic Compounds in Everyday Life Table Salt Covalent Bond The atoms in sugar do not form ions; Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Examples of compounds with ionic bonds include salt, such as table salt (nacl). Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1. Table Salt Covalent Bond.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Table Salt Covalent Bond Examples of compounds with ionic bonds include salt, such as table salt (nacl). Nevertheless, the compound has properties completely different from either elemental sodium. The atoms in sugar do not form ions; Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Sodium chloride also known as table salt, is. Table Salt Covalent Bond.
From cabinet.matttroy.net
Table Salt Nacl Is A Molecular Compound True Or False Matttroy Table Salt Covalent Bond Examples of compounds with ionic bonds include salt, such as table salt (nacl). The atoms in sugar do not form ions; Table sugar or sucrose differs from salt in the bonding between its atoms. This type of bonding occurs between two atoms of the same. An atom of sodium (na) donates one of its electrons to an atom of chlorine. Table Salt Covalent Bond.
From owlcation.com
Primary and Secondary Bonds Owlcation Table Salt Covalent Bond Covalent bonding is the sharing of electrons between atoms. Examples of compounds with ionic bonds include salt, such as table salt (nacl). This type of bonding occurs between two atoms of the same. Nevertheless, the compound has properties completely different from either elemental sodium. Table salt, as we have seen, consists of only two elements: Compounds composed of ions are. Table Salt Covalent Bond.
From www.vrogue.co
Covalent Bond Formed Example 5 Covalent Bonding Bond vrogue.co Table Salt Covalent Bond Examples of compounds with ionic bonds include salt, such as table salt (nacl). Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1 ratio of sodium. Table salt, as we have seen, consists of only two elements: Table sugar or sucrose differs from salt in the bonding between its atoms. The. Table Salt Covalent Bond.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID3665787 Table Salt Covalent Bond Table sugar or sucrose differs from salt in the bonding between its atoms. Covalent bonding is the sharing of electrons between atoms. In salt, the sodium atom donates its electron, so it yields the na + ion in water, while the. Nevertheless, the compound has properties completely different from either elemental sodium. Table salt, as we have seen, consists of. Table Salt Covalent Bond.
From readingandwritingprojectcom.web.fc2.com
the bond in table salt (nacl) is Table Salt Covalent Bond This type of bonding occurs between two atoms of the same. Examples of compounds with ionic bonds include salt, such as table salt (nacl). An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a stable ionic.. Table Salt Covalent Bond.
From chemistrylhs.weebly.com
GCSE ChemistryLHS Table Salt Covalent Bond An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a stable ionic. Table sugar or sucrose differs from salt in the bonding between its atoms. Examples of compounds with ionic bonds include salt, such as table. Table Salt Covalent Bond.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Table Salt Covalent Bond Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: The atoms in sugar do not form ions; An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −). Table Salt Covalent Bond.
From courses.lumenlearning.com
Covalent Bonds Biology for Majors I Table Salt Covalent Bond An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a stable ionic. In salt, the sodium atom donates its electron, so it yields the na + ion in water, while the. Table sugar or sucrose differs. Table Salt Covalent Bond.
From www.youtube.com
Is Table Salt (NaCl ) Ionic or Covalent/Molecular? YouTube Table Salt Covalent Bond Table salt, as we have seen, consists of only two elements: In salt, the sodium atom donates its electron, so it yields the na + ion in water, while the. Examples of compounds with ionic bonds include salt, such as table salt (nacl). This type of bonding occurs between two atoms of the same. Compounds composed of ions are called. Table Salt Covalent Bond.
From www.onlineworksheet.my.id
Ionic And Covalent Bonding Worksheet Onlineworksheet.my.id Table Salt Covalent Bond In salt, the sodium atom donates its electron, so it yields the na + ion in water, while the. This type of bonding occurs between two atoms of the same. Table salt, as we have seen, consists of only two elements: Table sugar or sucrose differs from salt in the bonding between its atoms. Nevertheless, the compound has properties completely. Table Salt Covalent Bond.
From www.slideshare.net
Chemistry of Life Table Salt Covalent Bond Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Nevertheless, the compound has properties completely different from either elemental sodium. The atoms in sugar do not form ions; Covalent bonding is the sharing of electrons between atoms. An atom of sodium (na) donates one of its electrons to an. Table Salt Covalent Bond.
From www.pinterest.com
1000+ images about Chem Bonding on Pinterest Science, Chemistry Table Salt Covalent Bond Covalent bonding is the sharing of electrons between atoms. The atoms in sugar do not form ions; Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1 ratio of sodium. In salt, the sodium atom donates its electron, so it yields the na + ion in water, while the. An atom. Table Salt Covalent Bond.
From www.vrogue.co
Covalent Vs Metallic Vs Ionic Bond General Difference vrogue.co Table Salt Covalent Bond Examples of compounds with ionic bonds include salt, such as table salt (nacl). Table sugar or sucrose differs from salt in the bonding between its atoms. This type of bonding occurs between two atoms of the same. Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1 ratio of sodium. Nevertheless,. Table Salt Covalent Bond.
From www.youtube.com
CHEMISTRY 101 Bond energies and bond lengths YouTube Table Salt Covalent Bond This type of bonding occurs between two atoms of the same. Table salt, as we have seen, consists of only two elements: Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a. Table Salt Covalent Bond.
From meaningkosh.com
Chemical Bond Definition MeaningKosh Table Salt Covalent Bond Table salt, as we have seen, consists of only two elements: Covalent bonding is the sharing of electrons between atoms. Table sugar or sucrose differs from salt in the bonding between its atoms. Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1 ratio of sodium. An atom of sodium (na). Table Salt Covalent Bond.
From www.numerade.com
Noncovalent interactions While the primary structure of TABLE 25 Four Table Salt Covalent Bond Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1 ratio of sodium. Table salt, as we have seen, consists of only two elements: Table sugar or sucrose differs from salt in the bonding between its atoms. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions. Table Salt Covalent Bond.
From www.baristahustle.com
TWC 0.02 How Does Water Dissolve Mineral Salts? Barista Hustle Table Salt Covalent Bond The atoms in sugar do not form ions; Covalent bonding is the sharing of electrons between atoms. Table salt, as we have seen, consists of only two elements: An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −). Table Salt Covalent Bond.
From www.slideserve.com
PPT Chapter 2 Molecules of Life PowerPoint Presentation, free Table Salt Covalent Bond Table sugar or sucrose differs from salt in the bonding between its atoms. Table salt, as we have seen, consists of only two elements: Nevertheless, the compound has properties completely different from either elemental sodium. The atoms in sugar do not form ions; Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together. Table Salt Covalent Bond.
From www.slideserve.com
PPT Table of Contents Atoms, Bonding, and the Periodic Table Ionic Table Salt Covalent Bond The atoms in sugar do not form ions; This type of bonding occurs between two atoms of the same. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Nevertheless, the compound has properties completely different from either elemental sodium. Sodium chloride also known as table salt, is an ionic. Table Salt Covalent Bond.
From byjus.com
Sodium Chloride Preparation, Properties, Structure & Uses Byju's Table Salt Covalent Bond Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1 ratio of sodium. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Covalent bonding is the sharing of electrons between atoms. An atom of sodium (na) donates one of its. Table Salt Covalent Bond.
From www.thoughtco.com
Chemical Composition of Table Salt Table Salt Covalent Bond This type of bonding occurs between two atoms of the same. Nevertheless, the compound has properties completely different from either elemental sodium. Table salt, as we have seen, consists of only two elements: Covalent bonding is the sharing of electrons between atoms. In salt, the sodium atom donates its electron, so it yields the na + ion in water, while. Table Salt Covalent Bond.
From pt.dreamstime.com
Covalente Ilustrações, Vetores E Clipart De Stock (649 Stock Table Salt Covalent Bond Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1 ratio of sodium. In salt, the sodium atom donates its electron, so it yields the na + ion in water, while the. Table sugar or sucrose differs from salt in the bonding between its atoms. Compounds composed of ions are called. Table Salt Covalent Bond.
From wou.edu
CH150 Chapter 7 Solutions Chemistry Table Salt Covalent Bond Nevertheless, the compound has properties completely different from either elemental sodium. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a stable ionic. In salt, the sodium atom donates its electron, so it yields the na. Table Salt Covalent Bond.
From www.slideserve.com
PPT Chemical Bonds PowerPoint Presentation, free download ID6126176 Table Salt Covalent Bond Examples of compounds with ionic bonds include salt, such as table salt (nacl). The atoms in sugar do not form ions; Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Covalent bonding is the sharing of electrons between atoms. Table salt, as we have seen, consists of only two. Table Salt Covalent Bond.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples Table Salt Covalent Bond Examples of compounds with ionic bonds include salt, such as table salt (nacl). Nevertheless, the compound has properties completely different from either elemental sodium. The atoms in sugar do not form ions; Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: This type of bonding occurs between two atoms. Table Salt Covalent Bond.
From www.vrogue.co
Periodic Table Of Elements Ionic Bonding Mrs Zeringue vrogue.co Table Salt Covalent Bond The atoms in sugar do not form ions; An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a stable ionic. Table salt, as we have seen, consists of only two elements: This type of bonding occurs. Table Salt Covalent Bond.
From thisonevsthatone.com
Ionic vs Covalent Which is which and how to tell them apart Table Salt Covalent Bond Nevertheless, the compound has properties completely different from either elemental sodium. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Table salt, as we have seen, consists of only two elements: The atoms in sugar do not form ions; An atom of sodium (na) donates one of its electrons. Table Salt Covalent Bond.
From mrtremblaycambridge.weebly.com
C11. Preparing common salts Mr. Tremblay's Class Site Table Salt Covalent Bond Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Covalent bonding is the sharing of electrons between atoms. Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1 ratio of sodium. The atoms in sugar do not form ions; Table. Table Salt Covalent Bond.
From www.studocu.com
Lab Report Ionic and Covalent Bonds NAME DATE Lab Report Ionic and Table Salt Covalent Bond In salt, the sodium atom donates its electron, so it yields the na + ion in water, while the. This type of bonding occurs between two atoms of the same. Sodium chloride also known as table salt, is an ionic compound with the chemical formula \(\ce{nacl}\), representing a 1:1 ratio of sodium. Compounds composed of ions are called ionic compounds. Table Salt Covalent Bond.
From brainly.com
This image shows the ionic bond between sodium and chloride in NaCl Table Salt Covalent Bond Table salt, as we have seen, consists of only two elements: An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a stable ionic. This type of bonding occurs between two atoms of the same. Covalent bonding. Table Salt Covalent Bond.
From studylib.net
Sugar or Salt? Ionic and Covalent Bonds Table Salt Covalent Bond Nevertheless, the compound has properties completely different from either elemental sodium. Covalent bonding is the sharing of electrons between atoms. This type of bonding occurs between two atoms of the same. Examples of compounds with ionic bonds include salt, such as table salt (nacl). An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl). Table Salt Covalent Bond.