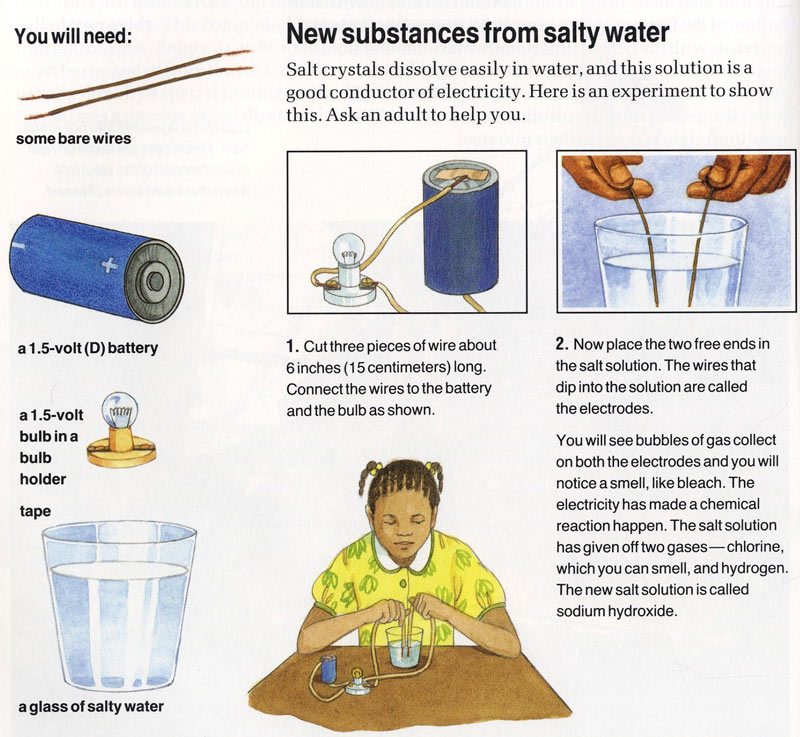
Can Nacl Conduct Electricity In Water . Pure water is not very conductive, and only a tiny bit of current can move through the water. Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. This means it yields ions in solution. Ions carry electric charges and therefore through. Why is distilled water a weaker conductor than tap water? When salt or sodium chloride (nacl) is dissolved in it, however, the. Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. Why an aqueous solution of nacl n a c l conducts electricity. Because nacl n a c l is an electrolyte. An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the.
from www.ency123.com
When salt or sodium chloride (nacl) is dissolved in it, however, the. Pure water is not very conductive, and only a tiny bit of current can move through the water. Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. Because nacl n a c l is an electrolyte. Why is distilled water a weaker conductor than tap water? Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. This means it yields ions in solution. Why an aqueous solution of nacl n a c l conducts electricity. Ions carry electric charges and therefore through.
Does Salt Water Conduct Electricity?
Can Nacl Conduct Electricity In Water Ions carry electric charges and therefore through. This means it yields ions in solution. Why is distilled water a weaker conductor than tap water? Ions carry electric charges and therefore through. Pure water is not very conductive, and only a tiny bit of current can move through the water. Because nacl n a c l is an electrolyte. An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. Why an aqueous solution of nacl n a c l conducts electricity. When salt or sodium chloride (nacl) is dissolved in it, however, the.
From materialmcgheepearter.z21.web.core.windows.net
Can Salt Water Conduct Electricity Can Nacl Conduct Electricity In Water When salt or sodium chloride (nacl) is dissolved in it, however, the. Ions carry electric charges and therefore through. Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. Pure water is not very conductive, and only a tiny bit of current can move through the water. Because nacl n a c l is an electrolyte.. Can Nacl Conduct Electricity In Water.
From www.slideserve.com
PPT Unit 4 Aqueous Reactions and Solution Stoichiometry PowerPoint Can Nacl Conduct Electricity In Water Ions carry electric charges and therefore through. This means it yields ions in solution. When salt or sodium chloride (nacl) is dissolved in it, however, the. Pure water is not very conductive, and only a tiny bit of current can move through the water. Because nacl n a c l is an electrolyte. Sodium chloride as a solid is not. Can Nacl Conduct Electricity In Water.
From www.teachoo.com
The table shown below gives information about four Chemistry MCQ Can Nacl Conduct Electricity In Water Ions carry electric charges and therefore through. Because nacl n a c l is an electrolyte. Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. This means it yields ions in solution. Pure water is not very conductive, and only a tiny bit of. Can Nacl Conduct Electricity In Water.
From mammothmemory.net
Electrolyte particles in the body transmitting electricity Can Nacl Conduct Electricity In Water Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. Ions carry electric charges and therefore through. Pure water is not very conductive, and only a tiny bit of current can move through the water. An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and. Can Nacl Conduct Electricity In Water.
From learningnadeaukarakas.z21.web.core.windows.net
Salt Water And Electrical Current Can Nacl Conduct Electricity In Water Pure water is not very conductive, and only a tiny bit of current can move through the water. Why an aqueous solution of nacl n a c l conducts electricity. Why is distilled water a weaker conductor than tap water? Because nacl n a c l is an electrolyte. Sodium chloride as a solid is not a good conductor of. Can Nacl Conduct Electricity In Water.
From www.youtube.com
Why Saltwater Conducts Electricity Science Experiment YouTube Can Nacl Conduct Electricity In Water This means it yields ions in solution. An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. Why an aqueous solution of nacl n a c l conducts electricity. Pure water is not very. Can Nacl Conduct Electricity In Water.
From www.researchgate.net
Comparison of the electrical conductivity of water and NaCl electrolyte Can Nacl Conduct Electricity In Water Because nacl n a c l is an electrolyte. Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. Pure water is not very conductive, and only a tiny bit of current can move through the water. Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water. Can Nacl Conduct Electricity In Water.
From techiescientist.com
Does Ice Conduct Electricity? Techiescientist Can Nacl Conduct Electricity In Water This means it yields ions in solution. Pure water is not very conductive, and only a tiny bit of current can move through the water. Because nacl n a c l is an electrolyte. Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. Ions carry electric charges and therefore through. Why is distilled water a. Can Nacl Conduct Electricity In Water.
From materialmcgheepearter.z21.web.core.windows.net
Can Salt Water Conduct Electricity Can Nacl Conduct Electricity In Water When salt or sodium chloride (nacl) is dissolved in it, however, the. Why is distilled water a weaker conductor than tap water? This means it yields ions in solution. Why an aqueous solution of nacl n a c l conducts electricity. Because nacl n a c l is an electrolyte. Sodium chloride is made of positively charged sodium ions and. Can Nacl Conduct Electricity In Water.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps Can Nacl Conduct Electricity In Water Ions carry electric charges and therefore through. Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. This means it yields ions in solution. Pure water is not very conductive, and only a tiny bit of current can move through the water. An ionic solid such as sodium chloride dissolves in water because of the electrostatic. Can Nacl Conduct Electricity In Water.
From www.slideshare.net
Chemical bonding Can Nacl Conduct Electricity In Water This means it yields ions in solution. Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. Pure water is not very conductive, and only a tiny bit of current can move through the. Can Nacl Conduct Electricity In Water.
From www.youtube.com
electrical conductivity with salt water science experiment working Can Nacl Conduct Electricity In Water Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. Ions carry electric charges and therefore through. Pure water is not very conductive, and only a tiny bit of current can. Can Nacl Conduct Electricity In Water.
From msestudent.com
Why Do Metals Conduct Electricity? Materials Science & Engineering Can Nacl Conduct Electricity In Water Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. When salt or sodium chloride (nacl) is dissolved in it, however, the. Pure water is not very conductive, and only a tiny bit of current can move through the water. An ionic solid such as. Can Nacl Conduct Electricity In Water.
From brainly.in
describe an activity to show that solution of salt in water can conduct Can Nacl Conduct Electricity In Water Why is distilled water a weaker conductor than tap water? When salt or sodium chloride (nacl) is dissolved in it, however, the. Why an aqueous solution of nacl n a c l conducts electricity. An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. Because nacl n a. Can Nacl Conduct Electricity In Water.
From materialmcgheepearter.z21.web.core.windows.net
Can Salt Water Conduct Electricity Can Nacl Conduct Electricity In Water Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. When salt or sodium chloride (nacl) is dissolved in it, however, the. Sodium chloride as a solid is not a good conductor of electricity. Can Nacl Conduct Electricity In Water.
From klasseddo.blob.core.windows.net
Can Gold Conduct Electricity In Water at Kurt Bruner blog Can Nacl Conduct Electricity In Water Pure water is not very conductive, and only a tiny bit of current can move through the water. Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. Why is distilled water a weaker conductor than tap water? Why an aqueous solution of nacl n. Can Nacl Conduct Electricity In Water.
From www.slideserve.com
PPT 1. What does this symbol mean? PowerPoint Presentation, free Can Nacl Conduct Electricity In Water Why an aqueous solution of nacl n a c l conducts electricity. Ions carry electric charges and therefore through. Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. Because nacl n a c l is an electrolyte. This means it yields ions in solution.. Can Nacl Conduct Electricity In Water.
From www.slideshare.net
Chemical bonding Can Nacl Conduct Electricity In Water Ions carry electric charges and therefore through. Why is distilled water a weaker conductor than tap water? This means it yields ions in solution. Pure water is not very conductive, and only a tiny bit of current can move through the water. When salt or sodium chloride (nacl) is dissolved in it, however, the. Because nacl n a c l. Can Nacl Conduct Electricity In Water.
From slideplayer.com
Chemical Bonding Chapter ppt download Can Nacl Conduct Electricity In Water Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. Pure water is not very conductive, and only a tiny bit of current can move through the water. An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. Because nacl n a c l. Can Nacl Conduct Electricity In Water.
From www.slideserve.com
PPT ELECTROLYSIS A guide for GCSE students PowerPoint Presentation Can Nacl Conduct Electricity In Water Ions carry electric charges and therefore through. Because nacl n a c l is an electrolyte. This means it yields ions in solution. Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. Why is distilled water a weaker conductor than tap water? Sodium chloride. Can Nacl Conduct Electricity In Water.
From www.slideserve.com
PPT Plan for Wed, 1 Oct 08 PowerPoint Presentation ID4479101 Can Nacl Conduct Electricity In Water An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. This means it yields ions in solution. Why an aqueous solution of nacl. Can Nacl Conduct Electricity In Water.
From www.rookieparenting.com
Does Water Conduct Electricity? Simple Experiment Can Nacl Conduct Electricity In Water When salt or sodium chloride (nacl) is dissolved in it, however, the. Why an aqueous solution of nacl n a c l conducts electricity. Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. Why is distilled water a weaker conductor than tap water? Pure water is not very conductive, and only a tiny bit of. Can Nacl Conduct Electricity In Water.
From decadethirty.com
Why Does Sodium Chloride Conduct Electricity? DECADE THIRTY Can Nacl Conduct Electricity In Water Ions carry electric charges and therefore through. Because nacl n a c l is an electrolyte. Pure water is not very conductive, and only a tiny bit of current can move through the water. Why is distilled water a weaker conductor than tap water? Sodium chloride as a solid is not a good conductor of electricity until it has been. Can Nacl Conduct Electricity In Water.
From slideplayer.com
Chapter 15 “Water and Aqueous Systems” ppt download Can Nacl Conduct Electricity In Water Pure water is not very conductive, and only a tiny bit of current can move through the water. Because nacl n a c l is an electrolyte. When salt or sodium chloride (nacl) is dissolved in it, however, the. This means it yields ions in solution. An ionic solid such as sodium chloride dissolves in water because of the electrostatic. Can Nacl Conduct Electricity In Water.
From www.youtube.com
Molten Sodium Chloride Conduct Electricity Why Sodium Chloride Conduct Can Nacl Conduct Electricity In Water Ions carry electric charges and therefore through. Because nacl n a c l is an electrolyte. Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. This means it yields ions in solution. When salt or sodium chloride (nacl) is dissolved in it, however, the.. Can Nacl Conduct Electricity In Water.
From loezuwgau.blob.core.windows.net
How To Accurately Measure The Electrical Conductivity Of A Liquid at Can Nacl Conduct Electricity In Water Why an aqueous solution of nacl n a c l conducts electricity. Because nacl n a c l is an electrolyte. An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. Pure water is not very conductive, and only a tiny bit of current can move through the. Can Nacl Conduct Electricity In Water.
From chemistrylabs-2.blogspot.com
Salt Water Electrical Conductivity Chemistry Labs Can Nacl Conduct Electricity In Water This means it yields ions in solution. Pure water is not very conductive, and only a tiny bit of current can move through the water. Why is distilled water a weaker conductor than tap water? Why an aqueous solution of nacl n a c l conducts electricity. Because nacl n a c l is an electrolyte. An ionic solid such. Can Nacl Conduct Electricity In Water.
From www.youtube.com
Electrical conductivity with salt water YouTube Can Nacl Conduct Electricity In Water Pure water is not very conductive, and only a tiny bit of current can move through the water. Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. Why is distilled water a weaker conductor than tap water? Why an aqueous solution of nacl n. Can Nacl Conduct Electricity In Water.
From materialmcgheepearter.z21.web.core.windows.net
Can Salt Water Conduct Electricity Can Nacl Conduct Electricity In Water Why is distilled water a weaker conductor than tap water? When salt or sodium chloride (nacl) is dissolved in it, however, the. Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. Because nacl n a c l is an electrolyte. Ions carry electric charges. Can Nacl Conduct Electricity In Water.
From www.ency123.com
Does Salt Water Conduct Electricity? Can Nacl Conduct Electricity In Water Pure water is not very conductive, and only a tiny bit of current can move through the water. When salt or sodium chloride (nacl) is dissolved in it, however, the. Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. This means it yields ions. Can Nacl Conduct Electricity In Water.
From sciencing.com
Why Salt in Water Can Conduct Electricity Sciencing Can Nacl Conduct Electricity In Water Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or has been melted to become. Because nacl n a c l is an electrolyte. Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. An ionic solid such as sodium chloride dissolves in water because of. Can Nacl Conduct Electricity In Water.
From tiantanstemcell.com
¿Por qué el NaCl acuoso conduce la electricidad? Tiantan Can Nacl Conduct Electricity In Water Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. When salt or sodium chloride (nacl) is dissolved in it, however, the. Why an aqueous solution of nacl n a c l conducts electricity. This means it yields ions in solution. An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction. Can Nacl Conduct Electricity In Water.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I Lewis Theory PowerPoint Can Nacl Conduct Electricity In Water Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. Ions carry electric charges and therefore through. This means it yields ions in solution. Why an aqueous solution of nacl n a c l conducts electricity. Because nacl n a c l is an electrolyte. Why is distilled water a weaker conductor than tap water? When. Can Nacl Conduct Electricity In Water.
From materialmcgheepearter.z21.web.core.windows.net
Can Salt Water Conduct Electricity Can Nacl Conduct Electricity In Water An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. Pure water is not very conductive, and only a tiny bit of current can move through the water. Sodium chloride as a solid is not a good conductor of electricity until it has been dissolved in water or. Can Nacl Conduct Electricity In Water.
From ceopyitw.blob.core.windows.net
What Compounds When Dissolved In Water Conduct Electricity at Jon Can Nacl Conduct Electricity In Water Pure water is not very conductive, and only a tiny bit of current can move through the water. An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (na +) and the. Sodium chloride is made of positively charged sodium ions and negatively charged choride ions. This means it yields ions in. Can Nacl Conduct Electricity In Water.