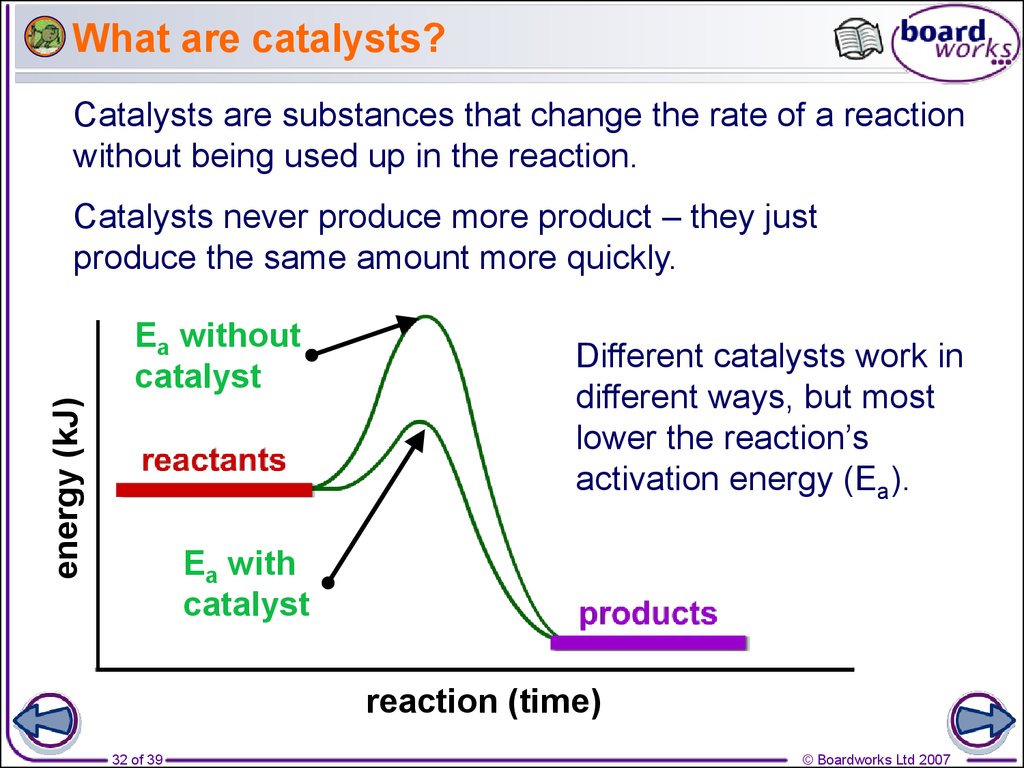
Catalysts In Rate Of Reaction . Only a very small mass of catalyst is needed to increase the rate of a reaction. Factors influencing the reaction rate: This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Adding a drop of blood to a. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It accomplishes this task by providing. However, not all reactions have suitable catalysts. Different substances catalyse different reactions. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Iron is a moderately reactive metal, meaning it readily participates in. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. It assumes familiarity with basic concepts in the collision.
from loevndwvy.blob.core.windows.net
Different substances catalyse different reactions. Adding a drop of blood to a. Iron is a moderately reactive metal, meaning it readily participates in. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. However, not all reactions have suitable catalysts. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Factors influencing the reaction rate: This page describes and explains the way that adding a catalyst affects the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction.
A Catalyst Increase The Rate Of A Reaction By at Carey Rains blog
Catalysts In Rate Of Reaction This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Adding a drop of blood to a. However, not all reactions have suitable catalysts. This page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision. Factors influencing the reaction rate: Iron is a moderately reactive metal, meaning it readily participates in. Different substances catalyse different reactions. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. It accomplishes this task by providing.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 Catalysts In Rate Of Reaction However, not all reactions have suitable catalysts. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Different substances catalyse different reactions. This page explains how adding a catalyst affects the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a. Catalysts In Rate Of Reaction.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalysts In Rate Of Reaction It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. Different substances catalyse different reactions. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Factors influencing the reaction rate: This page explains how adding a catalyst affects the rate of a reaction. Iron is a moderately. Catalysts In Rate Of Reaction.
From byjus.com
How does a catalyst increase the rate of a reaction? Catalysts In Rate Of Reaction Only a very small mass of catalyst is needed to increase the rate of a reaction. Iron is a moderately reactive metal, meaning it readily participates in. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids;. Catalysts In Rate Of Reaction.
From exoigvwpw.blob.core.windows.net
The Effect Of Catalysts On Rate Of Reaction at Clyde Berry blog Catalysts In Rate Of Reaction This page describes and explains the way that adding a catalyst affects the rate of a reaction. It accomplishes this task by providing. Different substances catalyse different reactions. This page explains how adding a catalyst affects the rate of a reaction. Adding a drop of blood to a. Only a very small mass of catalyst is needed to increase the. Catalysts In Rate Of Reaction.
From www.tes.com
Catalysts on the Rate of Reaction KS4 AQA Chemistry Teaching Resources Catalysts In Rate Of Reaction Only a very small mass of catalyst is needed to increase the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. Different substances catalyse different reactions. It assumes familiarity with basic concepts in the collision. A catalyst is a substance that increases the rate of a chemical reaction without being used up. Catalysts In Rate Of Reaction.
From slideplayer.com
Catalysts Rates of Reactions. ppt download Catalysts In Rate Of Reaction Adding a drop of blood to a. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Different substances catalyse different reactions. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. Only a very small mass of catalyst is needed to increase the rate of a. Catalysts In Rate Of Reaction.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Catalysts In Rate Of Reaction Factors influencing the reaction rate: This page describes and explains the way that adding a catalyst affects the rate of a reaction. Iron is a moderately reactive metal, meaning it readily participates in. It assumes familiarity with basic concepts in the collision. A catalyst is a substance that increases the rate of a chemical reaction without being used up in. Catalysts In Rate Of Reaction.
From www.slideshare.net
1.4 rate of reaction(1.2d)...biology Catalysts In Rate Of Reaction Different substances catalyse different reactions. Adding a drop of blood to a. Iron is a moderately reactive metal, meaning it readily participates in. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; It accomplishes this task by providing. A catalyst is a substance that increases the rate of a chemical reaction without being. Catalysts In Rate Of Reaction.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint Catalysts In Rate Of Reaction This page describes and explains the way that adding a catalyst affects the rate of a reaction. Iron is a moderately reactive metal, meaning it readily participates in. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Different substances catalyse different reactions. This page explains how adding a catalyst. Catalysts In Rate Of Reaction.
From loeljyzzv.blob.core.windows.net
Catalyst Chemical Reaction Rate at Leandro Jordan blog Catalysts In Rate Of Reaction This page explains how adding a catalyst affects the rate of a reaction. It accomplishes this task by providing. Adding a drop of blood to a. Different substances catalyse different reactions. Only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes familiarity with basic concepts in the collision. It assumes that you. Catalysts In Rate Of Reaction.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalysts In Rate Of Reaction Factors influencing the reaction rate: Only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This page explains how adding. Catalysts In Rate Of Reaction.
From exomdmjjp.blob.core.windows.net
Examples Of Catalysts Bbc Bitesize at Jacqueline Epp blog Catalysts In Rate Of Reaction Iron is a moderately reactive metal, meaning it readily participates in. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Different substances catalyse different reactions. However, not all reactions have suitable catalysts.. Catalysts In Rate Of Reaction.
From www.sasadoctor.com
Rate of reaction chemistry igcse Catalysts In Rate Of Reaction Factors influencing the reaction rate: The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. However, not all reactions have suitable catalysts. Different substances catalyse different reactions. This page explains how adding a catalyst. Catalysts In Rate Of Reaction.
From studyfullfarrior.z19.web.core.windows.net
Rates Of Reaction And Energy Changes Catalysts In Rate Of Reaction It assumes familiarity with basic concepts in the collision. Iron is a moderately reactive metal, meaning it readily participates in. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. Only a very small mass of catalyst is needed to increase the rate of a reaction. Different substances catalyse different reactions. A catalyst. Catalysts In Rate Of Reaction.
From study.com
Effect of Catalysts on Rates of Reaction Lesson Catalysts In Rate Of Reaction This page explains how adding a catalyst affects the rate of a reaction. Different substances catalyse different reactions. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Iron is a moderately reactive metal, meaning it readily participates in. The most effective catalyst of all is the enzyme catalase, present. Catalysts In Rate Of Reaction.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Catalysts In Rate Of Reaction Only a very small mass of catalyst is needed to increase the rate of a reaction. It accomplishes this task by providing. Factors influencing the reaction rate: It assumes familiarity with basic concepts in the collision. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. This page explains how. Catalysts In Rate Of Reaction.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts In Rate Of Reaction A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Different substances catalyse different reactions. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The most effective catalyst of. Catalysts In Rate Of Reaction.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the Catalysts In Rate Of Reaction It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. However, not all reactions have suitable catalysts. Different substances catalyse different reactions. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Iron. Catalysts In Rate Of Reaction.
From moicaucachep.com
How Catalysts Boost Reaction Rates Unveiling With Potential Energy Catalysts In Rate Of Reaction Different substances catalyse different reactions. Factors influencing the reaction rate: It accomplishes this task by providing. Iron is a moderately reactive metal, meaning it readily participates in. It assumes familiarity with basic concepts in the collision. This page explains how adding a catalyst affects the rate of a reaction. However, not all reactions have suitable catalysts. A catalyst is a. Catalysts In Rate Of Reaction.
From www.youtube.com
Factors Affecting the Rate of Reaction Catalyst Rate of Reaction Catalysts In Rate Of Reaction Different substances catalyse different reactions. Adding a drop of blood to a. Only a very small mass of catalyst is needed to increase the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance that increases the rate of a chemical reaction without being. Catalysts In Rate Of Reaction.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID Catalysts In Rate Of Reaction This page explains how adding a catalyst affects the rate of a reaction. Factors influencing the reaction rate: It assumes familiarity with basic concepts in the collision. Adding a drop of blood to a. Different substances catalyse different reactions. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. This page describes and. Catalysts In Rate Of Reaction.
From www.youtube.com
How Catalysts Affect Rate Of Reaction GCSE Chemistry Catalysts In Rate Of Reaction Different substances catalyse different reactions. However, not all reactions have suitable catalysts. Factors influencing the reaction rate: The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; It accomplishes this task by providing. It assumes familiarity with basic concepts in the collision. Iron is a moderately reactive metal, meaning it readily participates in. It. Catalysts In Rate Of Reaction.
From loevndwvy.blob.core.windows.net
A Catalyst Increase The Rate Of A Reaction By at Carey Rains blog Catalysts In Rate Of Reaction Only a very small mass of catalyst is needed to increase the rate of a reaction. Factors influencing the reaction rate: It accomplishes this task by providing. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. It assumes that. Catalysts In Rate Of Reaction.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 Catalysts In Rate Of Reaction Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; This page describes and explains the way that adding a catalyst affects the rate of a reaction. Adding a drop of. Catalysts In Rate Of Reaction.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalysts In Rate Of Reaction Adding a drop of blood to a. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Iron is a moderately reactive metal, meaning it readily participates in. Different substances catalyse different reactions. It accomplishes this task by providing. Factors influencing the reaction rate: Only a very small mass of. Catalysts In Rate Of Reaction.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalysts In Rate Of Reaction It assumes familiarity with basic concepts in the collision. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. This page explains how adding a catalyst affects the rate of. Catalysts In Rate Of Reaction.
From slideplayer.com
Catalysts Rates of Reactions. ppt download Catalysts In Rate Of Reaction Adding a drop of blood to a. This page explains how adding a catalyst affects the rate of a reaction. It accomplishes this task by providing. It assumes familiarity with basic concepts in the collision. Only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes that you are already familiar with basic. Catalysts In Rate Of Reaction.
From www.savemyexams.com
Catalysts & Rate of Reactions Oxford AQA IGCSE Chemistry Revision Catalysts In Rate Of Reaction This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. Iron is a moderately reactive metal, meaning it readily participates in. It accomplishes. Catalysts In Rate Of Reaction.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Catalysts In Rate Of Reaction The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; It accomplishes this task by providing. This page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. However, not all reactions have suitable catalysts. Adding. Catalysts In Rate Of Reaction.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation Catalysts In Rate Of Reaction The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Adding a drop of blood to a. However, not all reactions have suitable catalysts. This page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision. Iron is a moderately reactive metal, meaning it. Catalysts In Rate Of Reaction.
From exydadzyq.blob.core.windows.net
Catalyst Graph Chemistry at Margaret Wadlington blog Catalysts In Rate Of Reaction Iron is a moderately reactive metal, meaning it readily participates in. It assumes familiarity with basic concepts in the collision. Only a very small mass of catalyst is needed to increase the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Different substances catalyse different reactions. This page. Catalysts In Rate Of Reaction.
From www.slideserve.com
PPT Catalysts and Rate of Reaction PowerPoint Presentation, free Catalysts In Rate Of Reaction Iron is a moderately reactive metal, meaning it readily participates in. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Different substances catalyse different reactions. This page describes and explains the way that. Catalysts In Rate Of Reaction.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalysts In Rate Of Reaction This page describes and explains the way that adding a catalyst affects the rate of a reaction. Iron is a moderately reactive metal, meaning it readily participates in. However, not all reactions have suitable catalysts. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; It assumes that you are already familiar with basic. Catalysts In Rate Of Reaction.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalysts In Rate Of Reaction Factors influencing the reaction rate: Only a very small mass of catalyst is needed to increase the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. However, not all reactions have suitable catalysts. Different substances catalyse different reactions. The most effective catalyst of all is the enzyme catalase,. Catalysts In Rate Of Reaction.
From joifdozvf.blob.core.windows.net
What Is A Catalyst In Chemistry Example at Herbert Simpson blog Catalysts In Rate Of Reaction It assumes familiarity with basic concepts in the collision. This page explains how adding a catalyst affects the rate of a reaction. Factors influencing the reaction rate: Only a very small mass of catalyst is needed to increase the rate of a reaction. Iron is a moderately reactive metal, meaning it readily participates in. This page describes and explains the. Catalysts In Rate Of Reaction.