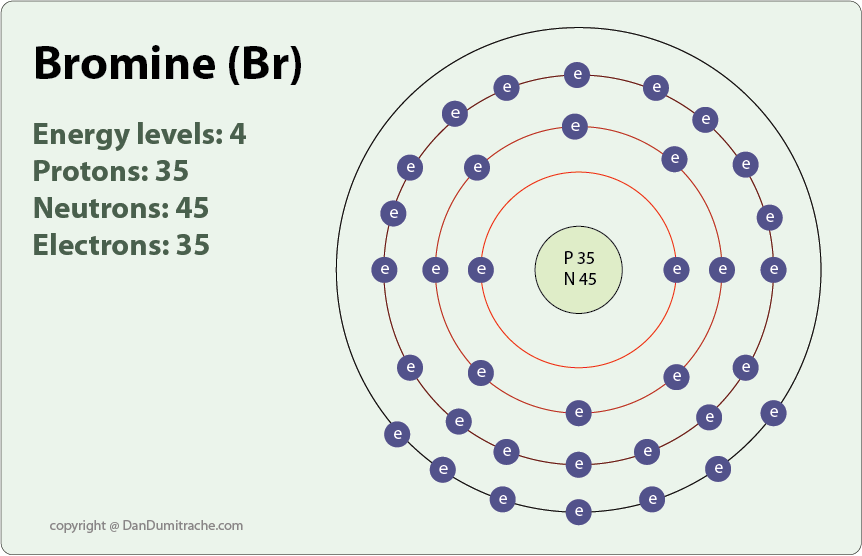
Fluorine Bromine Reaction . What about fluorine (f2), bromine (br2), and iodine (i2)? Fluorine we’ll leave until the end. this can be shown by looking at displacement reactions. Let’s look at bromine and iodine. Chlorine isn’t the only halogen on the periodic table. only the reactions of chlorine, bromine, and iodine can be considered. You can see the trend in reactivity if you react the halogens with iron wool. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. one last slide. fluorine is the most reactive element of all in group 7. Example when chlorine (as a gas or dissolved in water) is. Aqueous fluorine is very reactive with. one of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. The order of reactivity is.
from socratic.org
Example when chlorine (as a gas or dissolved in water) is. You can see the trend in reactivity if you react the halogens with iron wool. Let’s look at bromine and iodine. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. fluorine is the most reactive element of all in group 7. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. What about fluorine (f2), bromine (br2), and iodine (i2)? Chlorine isn’t the only halogen on the periodic table. Fluorine we’ll leave until the end. one last slide.
Why does fluorine have a higher ionization energy than bromine? Socratic
Fluorine Bromine Reaction one last slide. The order of reactivity is. Example when chlorine (as a gas or dissolved in water) is. Aqueous fluorine is very reactive with. one last slide. only the reactions of chlorine, bromine, and iodine can be considered. Chlorine isn’t the only halogen on the periodic table. Fluorine we’ll leave until the end. You can see the trend in reactivity if you react the halogens with iron wool. one of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. Let’s look at bromine and iodine. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. fluorine is the most reactive element of all in group 7. this can be shown by looking at displacement reactions. What about fluorine (f2), bromine (br2), and iodine (i2)?
From www.masterorganicchemistry.com
Alkyl Halide Reaction Map 14 Key Reactions Of Alkyl Halides Fluorine Bromine Reaction one last slide. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. fluorine is the most reactive element of all in group 7. Example when chlorine (as a gas or dissolved in water) is. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels. Fluorine Bromine Reaction.
From www.gauthmath.com
Fluorine reacts with sodium bromide in a single replacement reaction Fluorine Bromine Reaction all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. Fluorine we’ll leave until the end. one last slide. Example when chlorine (as a gas or dissolved in water) is. fluorine is the most reactive element of all in group 7. one of these reactions is halogenation, or the substitution of a. Fluorine Bromine Reaction.
From ar.inspiredpencil.com
Bromine Liquid Formula Fluorine Bromine Reaction Example when chlorine (as a gas or dissolved in water) is. The order of reactivity is. one of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to. use the results in the table to deduce an order of reactivity, starting with the most. Fluorine Bromine Reaction.
From www.dreamstime.com
Bromine Fluoride Molecule Stock Image Image 8439131 Fluorine Bromine Reaction Let’s look at bromine and iodine. this can be shown by looking at displacement reactions. fluorine is the most reactive element of all in group 7. You can see the trend in reactivity if you react the halogens with iron wool. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. Aqueous fluorine. Fluorine Bromine Reaction.
From www.dreamstime.com
Bromine fluoride stock illustration. Illustration of molecular 8439119 Fluorine Bromine Reaction You can see the trend in reactivity if you react the halogens with iron wool. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. this can be shown by looking at displacement reactions. Fluorine. Fluorine Bromine Reaction.
From www.tessshebaylo.com
Chemical Equation For Single Replacement Reaction Between Lithium Fluorine Bromine Reaction The order of reactivity is. Example when chlorine (as a gas or dissolved in water) is. fluorine is the most reactive element of all in group 7. this can be shown by looking at displacement reactions. What about fluorine (f2), bromine (br2), and iodine (i2)? You can see the trend in reactivity if you react the halogens with. Fluorine Bromine Reaction.
From www.tessshebaylo.com
Chemical Equation For Single Replacement Reaction Between Lithium Fluorine Bromine Reaction one last slide. Example when chlorine (as a gas or dissolved in water) is. Aqueous fluorine is very reactive with. only the reactions of chlorine, bromine, and iodine can be considered. What about fluorine (f2), bromine (br2), and iodine (i2)? this can be shown by looking at displacement reactions. Chlorine isn’t the only halogen on the periodic. Fluorine Bromine Reaction.
From www.chegg.com
Solved Alkenes are more reactive than benzene and undergo Fluorine Bromine Reaction only the reactions of chlorine, bromine, and iodine can be considered. Chlorine isn’t the only halogen on the periodic table. Example when chlorine (as a gas or dissolved in water) is. fluorine is the most reactive element of all in group 7. one last slide. Aqueous fluorine is very reactive with. Let’s look at bromine and iodine.. Fluorine Bromine Reaction.
From www.chemistrysteps.com
Preparation of Amines Chemistry Steps Fluorine Bromine Reaction one last slide. You can see the trend in reactivity if you react the halogens with iron wool. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. Chlorine isn’t the only halogen on the. Fluorine Bromine Reaction.
From trendrep.net
Bromering och oxidering av bensylkarboner Master Organic Chemistry Fluorine Bromine Reaction Example when chlorine (as a gas or dissolved in water) is. You can see the trend in reactivity if you react the halogens with iron wool. Let’s look at bromine and iodine. only the reactions of chlorine, bromine, and iodine can be considered. The order of reactivity is. fluorine is the most reactive element of all in group. Fluorine Bromine Reaction.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Fluorine Bromine Reaction The order of reactivity is. one last slide. Let’s look at bromine and iodine. this can be shown by looking at displacement reactions. What about fluorine (f2), bromine (br2), and iodine (i2)? all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. You can see the trend in reactivity if you react the. Fluorine Bromine Reaction.
From nap.nationalacademies.org
Table of Isotopes of Fluorine, Chlorine, Bromine and Iodine The Fluorine Bromine Reaction one of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to. fluorine is the most reactive element of all in group 7. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. Let’s look at bromine and iodine.. Fluorine Bromine Reaction.
From www.youtube.com
Reaction of Sodium fluoride with Bromine 38 YouTube Fluorine Bromine Reaction Example when chlorine (as a gas or dissolved in water) is. Fluorine we’ll leave until the end. Chlorine isn’t the only halogen on the periodic table. this can be shown by looking at displacement reactions. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. all the halogens react. Fluorine Bromine Reaction.
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic Fluorine Bromine Reaction this can be shown by looking at displacement reactions. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. Let’s look at bromine and iodine. You can see the trend in reactivity if you react the halogens with iron wool. What about fluorine (f2), bromine (br2), and iodine (i2)? The order of reactivity is.. Fluorine Bromine Reaction.
From www.youtube.com
Reaction of Bromine with Sodium YouTube Fluorine Bromine Reaction fluorine is the most reactive element of all in group 7. Example when chlorine (as a gas or dissolved in water) is. You can see the trend in reactivity if you react the halogens with iron wool. Chlorine isn’t the only halogen on the periodic table. Fluorine we’ll leave until the end. Let’s look at bromine and iodine. Aqueous. Fluorine Bromine Reaction.
From www.masterorganicchemistry.com
Making Alkyl Halides From Alcohols Master Organic Chemistry Fluorine Bromine Reaction use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. Aqueous fluorine is very reactive with. one last slide. Let’s look at bromine and iodine. Chlorine isn’t the only halogen on the periodic table. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. . Fluorine Bromine Reaction.
From ecurrencythailand.com
Which Is The Least Reactive Fluorine Chlorine Bromine Iodine? 22 Most Fluorine Bromine Reaction use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. Let’s look at bromine and iodine. one of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to. You can see the trend in reactivity if. Fluorine Bromine Reaction.
From www.researchgate.net
Iron (III) fluoride/SIPrcatalyzed cross coupling reaction of aryl Fluorine Bromine Reaction Aqueous fluorine is very reactive with. fluorine is the most reactive element of all in group 7. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. Example when chlorine (as a gas or dissolved in water) is. one of these reactions is halogenation, or the substitution of a. Fluorine Bromine Reaction.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Fluorine Bromine Reaction fluorine is the most reactive element of all in group 7. What about fluorine (f2), bromine (br2), and iodine (i2)? one last slide. Let’s look at bromine and iodine. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. Fluorine we’ll leave until the end. use the results in the table to. Fluorine Bromine Reaction.
From www.numerade.com
SOLVED Based on your observations, predict the reaction of bromide Fluorine Bromine Reaction The order of reactivity is. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. What about fluorine (f2), bromine (br2), and iodine (i2)? Let’s look at bromine and iodine. Fluorine we’ll leave until the end. only the reactions of chlorine, bromine, and iodine can be considered. one of these reactions is halogenation,. Fluorine Bromine Reaction.
From www.numerade.com
SOLVED What volume (in mL) of fluorine gas is required t0 react with 3 Fluorine Bromine Reaction use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. Fluorine we’ll leave until the end. this can be shown by looking at displacement reactions. fluorine is the most reactive element of all in group 7. What about fluorine (f2), bromine (br2), and iodine (i2)? only the reactions. Fluorine Bromine Reaction.
From www.tessshebaylo.com
Chemical Equation For Single Replacement Reaction Between Lithium Fluorine Bromine Reaction one last slide. Fluorine we’ll leave until the end. fluorine is the most reactive element of all in group 7. You can see the trend in reactivity if you react the halogens with iron wool. this can be shown by looking at displacement reactions. Example when chlorine (as a gas or dissolved in water) is. The order. Fluorine Bromine Reaction.
From www.haikudeck.com
Science by olga092464 Fluorine Bromine Reaction Let’s look at bromine and iodine. Fluorine we’ll leave until the end. Aqueous fluorine is very reactive with. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. one of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to.. Fluorine Bromine Reaction.
From www.researchgate.net
(PDF) Reactions of Bromine Fluoride Dioxide, BrO2F Fluorine Bromine Reaction all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. Fluorine we’ll leave until the end. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. The order of reactivity is. one of these reactions is halogenation, or the substitution of a single hydrogen on. Fluorine Bromine Reaction.
From www.alamy.com
halogens in gas jars Fl Cl Br I fluorine chlorine bromine iodine Stock Fluorine Bromine Reaction this can be shown by looking at displacement reactions. What about fluorine (f2), bromine (br2), and iodine (i2)? Aqueous fluorine is very reactive with. one of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to. fluorine is the most reactive element of. Fluorine Bromine Reaction.
From ar.inspiredpencil.com
Fluorine Molecular Structure Fluorine Bromine Reaction one last slide. What about fluorine (f2), bromine (br2), and iodine (i2)? Chlorine isn’t the only halogen on the periodic table. The order of reactivity is. Aqueous fluorine is very reactive with. Fluorine we’ll leave until the end. fluorine is the most reactive element of all in group 7. You can see the trend in reactivity if you. Fluorine Bromine Reaction.
From pubs.rsc.org
Facile onepot synthesis of sulfonyl fluorides from sulfonates or Fluorine Bromine Reaction Example when chlorine (as a gas or dissolved in water) is. only the reactions of chlorine, bromine, and iodine can be considered. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. You can see the trend in reactivity if you react the halogens with iron wool. What about fluorine. Fluorine Bromine Reaction.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Fluorine Bromine Reaction one last slide. Example when chlorine (as a gas or dissolved in water) is. You can see the trend in reactivity if you react the halogens with iron wool. one of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to. Fluorine we’ll leave. Fluorine Bromine Reaction.
From exofhdgch.blob.core.windows.net
Bromine Ionic Or Covalent at Donna McMillian blog Fluorine Bromine Reaction The order of reactivity is. Example when chlorine (as a gas or dissolved in water) is. all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of. You can see the trend in reactivity if you react the halogens with iron wool. one of these reactions is halogenation, or the substitution of a single hydrogen. Fluorine Bromine Reaction.
From depositphotos.com
Bromine fluoride molecule — Stock Photo © jbouzou 1885785 Fluorine Bromine Reaction You can see the trend in reactivity if you react the halogens with iron wool. one of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to. use the results in the table to deduce an order of reactivity, starting with the most reactive. Fluorine Bromine Reaction.
From brainly.in
Reaction between sodium iodide and bromine water Brainly.in Fluorine Bromine Reaction The order of reactivity is. Example when chlorine (as a gas or dissolved in water) is. What about fluorine (f2), bromine (br2), and iodine (i2)? only the reactions of chlorine, bromine, and iodine can be considered. this can be shown by looking at displacement reactions. Let’s look at bromine and iodine. Aqueous fluorine is very reactive with. . Fluorine Bromine Reaction.
From pubs.rsc.org
Electrophilic N trifluoromethylthiophthalimide as a fluorinated Fluorine Bromine Reaction only the reactions of chlorine, bromine, and iodine can be considered. You can see the trend in reactivity if you react the halogens with iron wool. Chlorine isn’t the only halogen on the periodic table. this can be shown by looking at displacement reactions. The order of reactivity is. one of these reactions is halogenation, or the. Fluorine Bromine Reaction.
From chehesj.blogspot.com
organic chemistry Why are fluorides more reactive in nucleophilic Fluorine Bromine Reaction this can be shown by looking at displacement reactions. one of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. You can see the. Fluorine Bromine Reaction.
From www.youtube.com
How to Balance F2 + LiBr = LiF + Br2 (Fluorine gas + Lithium bromide Fluorine Bromine Reaction You can see the trend in reactivity if you react the halogens with iron wool. one of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to. Chlorine isn’t the only halogen on the periodic table. one last slide. What about fluorine (f2), bromine. Fluorine Bromine Reaction.
From www.chegg.com
Solved We have seen that the SN2 reaction of ethyl bromide Fluorine Bromine Reaction What about fluorine (f2), bromine (br2), and iodine (i2)? You can see the trend in reactivity if you react the halogens with iron wool. Chlorine isn’t the only halogen on the periodic table. one last slide. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. Fluorine we’ll leave until. Fluorine Bromine Reaction.