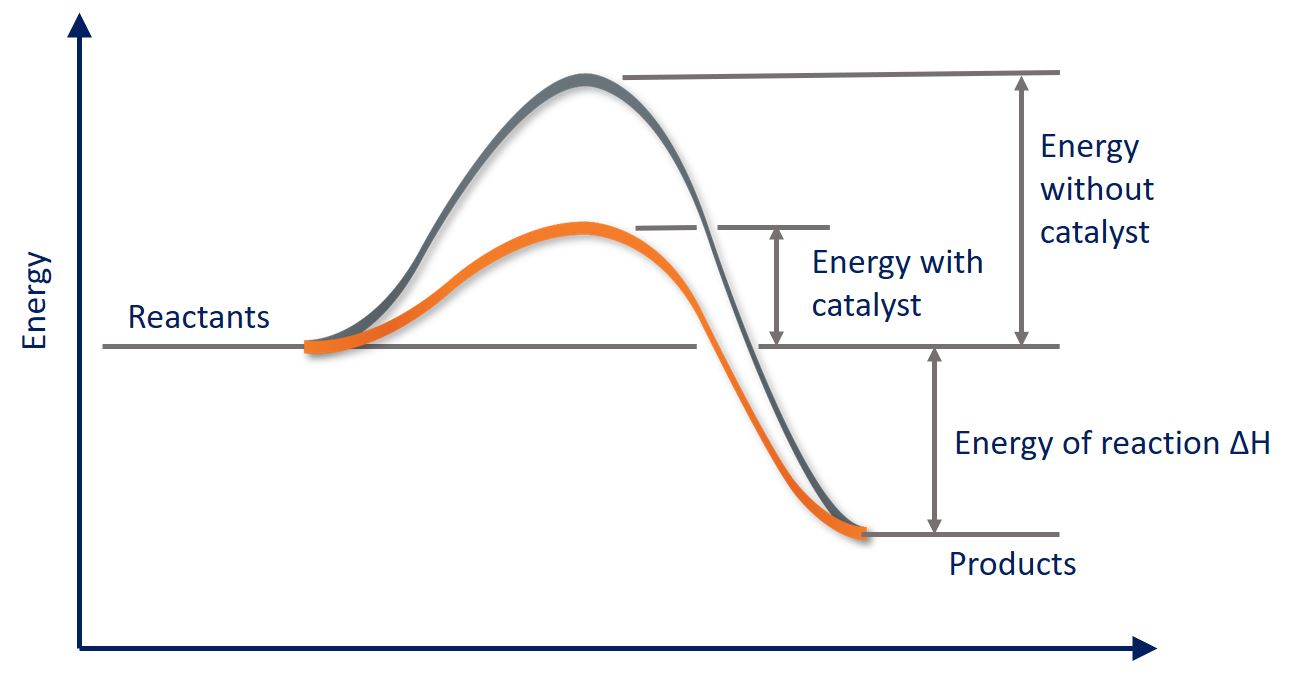
Reaction Graph With Catalyst . In this section, we will examine the three major classes of catalysts: In a heterogeneous reaction, the catalyst is in a different phase. One possible way of doing this is to provide an alternative way for. Reaction diagrams for a chemical process with and without a catalyst are shown below. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. To increase the rate of a reaction you need to increase the number of successful collisions. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Heterogeneous catalysts, homogeneous catalysts, and enzymes. In this section, we will examine the three major classes of catalysts: Lowering the activation energy of a reaction by a catalyst. Heterogeneous catalysts, homogeneous catalysts, and enzymes. This page explains how adding a catalyst affects the rate of a reaction. The catalyzed pathway involves a.
from www.catalystseurope.org
In this section, we will examine the three major classes of catalysts: In a heterogeneous reaction, the catalyst is in a different phase. One possible way of doing this is to provide an alternative way for. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Reaction diagrams for a chemical process with and without a catalyst are shown below. Heterogeneous catalysts, homogeneous catalysts, and enzymes. This page explains how adding a catalyst affects the rate of a reaction. The catalyzed pathway involves a. Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end.
What are catalysts?
Reaction Graph With Catalyst A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. In a heterogeneous reaction, the catalyst is in a different phase. To increase the rate of a reaction you need to increase the number of successful collisions. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. In this section, we will examine the three major classes of catalysts: Lowering the activation energy of a reaction by a catalyst. This page explains how adding a catalyst affects the rate of a reaction. Reaction diagrams for a chemical process with and without a catalyst are shown below. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Heterogeneous catalysts, homogeneous catalysts, and enzymes. In this section, we will examine the three major classes of catalysts: The catalyzed pathway involves a. One possible way of doing this is to provide an alternative way for.
From ar.inspiredpencil.com
Exothermic Reaction With Catalyst Reaction Graph With Catalyst Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. In a heterogeneous reaction, the catalyst is in a different phase. Heterogeneous catalysts, homogeneous catalysts, and enzymes. The catalyzed pathway involves a. Reaction diagrams for a chemical process with and without a catalyst are shown below. To increase the rate of a. Reaction Graph With Catalyst.
From schoolbag.info
CATALYSTS AND ENERGY DIAGRAMS Chemical Reactions, Energy Changes, and Reaction Graph With Catalyst Lowering the activation energy of a reaction by a catalyst. In this section, we will examine the three major classes of catalysts: This page explains how adding a catalyst affects the rate of a reaction. Reaction diagrams for a chemical process with and without a catalyst are shown below. A catalyst is a substance which speeds up a reaction, but. Reaction Graph With Catalyst.
From www.numerade.com
SOLVED The following set of graphs has exothermic and endothermic Reaction Graph With Catalyst In this section, we will examine the three major classes of catalysts: To increase the rate of a reaction you need to increase the number of successful collisions. This page explains how adding a catalyst affects the rate of a reaction. Reaction diagrams for a chemical process with and without a catalyst are shown below. A catalyst is a substance. Reaction Graph With Catalyst.
From chemistryguru.com.sg
Rate Concentration Graph for Enzyme Catalysed Reaction Reaction Graph With Catalyst In a heterogeneous reaction, the catalyst is in a different phase. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Heterogeneous catalysts, homogeneous catalysts, and enzymes. In this section, we will examine the three major classes of catalysts: A catalyst is a substance which speeds. Reaction Graph With Catalyst.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Reaction Graph With Catalyst Heterogeneous catalysts, homogeneous catalysts, and enzymes. In a heterogeneous reaction, the catalyst is in a different phase. The catalyzed pathway involves a. Lowering the activation energy of a reaction by a catalyst. This page explains how adding a catalyst affects the rate of a reaction. Heterogeneous catalysts, homogeneous catalysts, and enzymes. One possible way of doing this is to provide. Reaction Graph With Catalyst.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Reaction Graph With Catalyst To increase the rate of a reaction you need to increase the number of successful collisions. In this section, we will examine the three major classes of catalysts: Lowering the activation energy of a reaction by a catalyst. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. This page explains how. Reaction Graph With Catalyst.
From www.numerade.com
SOLVEDUse graphs to illustrate how the presence of a catalyst can Reaction Graph With Catalyst To increase the rate of a reaction you need to increase the number of successful collisions. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Lowering the activation energy of a reaction by a catalyst. In a heterogeneous reaction, the catalyst is in a different phase. The catalyzed pathway involves a. In this section, we will examine the three major classes of catalysts:. Reaction Graph With Catalyst.
From www.kentchemistry.com
Factors Affecting Reaction Rates Reaction Graph With Catalyst A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. Reaction diagrams for a chemical process with and without a catalyst are shown below. One possible way of doing this is to provide an alternative way for. In this section, we will examine the three major classes of catalysts: This page explains how. Reaction Graph With Catalyst.
From sites.google.com
New 6.0 DP CHEMISTRY Reaction Graph With Catalyst This page explains how adding a catalyst affects the rate of a reaction. The catalyzed pathway involves a. In this section, we will examine the three major classes of catalysts: Heterogeneous catalysts, homogeneous catalysts, and enzymes. In a heterogeneous reaction, the catalyst is in a different phase. One possible way of doing this is to provide an alternative way for.. Reaction Graph With Catalyst.
From usermanualwhelming.z21.web.core.windows.net
Reaction Profile Diagram With Catalyst Reaction Graph With Catalyst Reaction diagrams for a chemical process with and without a catalyst are shown below. Heterogeneous catalysts, homogeneous catalysts, and enzymes. To increase the rate of a reaction you need to increase the number of successful collisions. Lowering the activation energy of a reaction by a catalyst. This page explains how adding a catalyst affects the rate of a reaction. In. Reaction Graph With Catalyst.
From nonsibihighschool.org
Section 184 Catalysis Reaction Graph With Catalyst Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. Lowering the activation energy of a reaction by a catalyst. In this section, we will examine the three major classes of catalysts: In a heterogeneous reaction, the catalyst is in a different phase. Heterogeneous catalysts, homogeneous catalysts,. Reaction Graph With Catalyst.
From www.catalystseurope.org
What are catalysts? Reaction Graph With Catalyst In this section, we will examine the three major classes of catalysts: Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. In a heterogeneous reaction, the catalyst is. Reaction Graph With Catalyst.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Reaction Graph With Catalyst To increase the rate of a reaction you need to increase the number of successful collisions. Lowering the activation energy of a reaction by a catalyst. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Reaction diagrams for a chemical process with and without a catalyst are shown below. In a. Reaction Graph With Catalyst.
From courses.lumenlearning.com
Catalysis Chemistry Reaction Graph With Catalyst This page explains how adding a catalyst affects the rate of a reaction. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. One possible way of doing this is to provide an alternative way for. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Reaction diagrams for a chemical process with and without a. Reaction Graph With Catalyst.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Reaction Graph With Catalyst In this section, we will examine the three major classes of catalysts: In this section, we will examine the three major classes of catalysts: Reaction diagrams for a chemical process with and without a catalyst are shown below. This page explains how adding a catalyst affects the rate of a reaction. Reaction diagrams for an endothermic process in the absence. Reaction Graph With Catalyst.
From www.sliderbase.com
Endothermic Reaction w/Catalyst Reaction Graph With Catalyst Heterogeneous catalysts, homogeneous catalysts, and enzymes. The catalyzed pathway involves a. In this section, we will examine the three major classes of catalysts: A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. One possible way of doing this is to provide an alternative way for. Reaction diagrams for a chemical process with. Reaction Graph With Catalyst.
From www.youtube.com
Equilibrium Graphs grade 12 Catalyst YouTube Reaction Graph With Catalyst In this section, we will examine the three major classes of catalysts: This page explains how adding a catalyst affects the rate of a reaction. In this section, we will examine the three major classes of catalysts: In a heterogeneous reaction, the catalyst is in a different phase. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Reaction diagrams for a chemical process. Reaction Graph With Catalyst.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Reaction Graph With Catalyst A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. In this section, we will examine the three major classes of catalysts: To increase the rate of a reaction you need to increase the number of successful collisions. In a heterogeneous reaction, the catalyst is in a different phase. In this section, we. Reaction Graph With Catalyst.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint Reaction Graph With Catalyst In a heterogeneous reaction, the catalyst is in a different phase. This page explains how adding a catalyst affects the rate of a reaction. Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. To increase the rate of a reaction you need to increase the number. Reaction Graph With Catalyst.
From www.workmanagementsolutions.com.au
An Analogy The Activation Energy and Catalysis in Business Operations Reaction Graph With Catalyst This page explains how adding a catalyst affects the rate of a reaction. In a heterogeneous reaction, the catalyst is in a different phase. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Heterogeneous catalysts, homogeneous catalysts, and enzymes. The catalyzed pathway involves a. Reaction diagrams for a chemical process with and without a catalyst are shown below. In this section, we will. Reaction Graph With Catalyst.
From chem.libretexts.org
31.10 The HaberBosch Reaction Can Be Surface Catalyzed Chemistry Reaction Graph With Catalyst Lowering the activation energy of a reaction by a catalyst. Heterogeneous catalysts, homogeneous catalysts, and enzymes. One possible way of doing this is to provide an alternative way for. In this section, we will examine the three major classes of catalysts: In a heterogeneous reaction, the catalyst is in a different phase. The catalyzed pathway involves a. Reaction diagrams for. Reaction Graph With Catalyst.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Reaction Graph With Catalyst Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. To increase the rate of a reaction you need to increase the number of successful collisions. One possible way of doing this is to provide an alternative way for. In this section, we will examine the three major classes of catalysts: The. Reaction Graph With Catalyst.
From www.chemistrylearner.com
Exergonic Reaction Definition, Equation, Graph, and Examples Reaction Graph With Catalyst This page explains how adding a catalyst affects the rate of a reaction. In this section, we will examine the three major classes of catalysts: The catalyzed pathway involves a. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Lowering the activation energy of a reaction by a catalyst. Reaction diagrams for a chemical process with and. Reaction Graph With Catalyst.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Reaction Graph With Catalyst In this section, we will examine the three major classes of catalysts: Heterogeneous catalysts, homogeneous catalysts, and enzymes. One possible way of doing this is to provide an alternative way for. This page explains how adding a catalyst affects the rate of a reaction. In a heterogeneous reaction, the catalyst is in a different phase. Reaction diagrams for a chemical. Reaction Graph With Catalyst.
From ar.inspiredpencil.com
Catalyst Chemistry Reaction Graph With Catalyst The catalyzed pathway involves a. One possible way of doing this is to provide an alternative way for. Lowering the activation energy of a reaction by a catalyst. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. In a heterogeneous reaction, the catalyst is in a different phase. Heterogeneous catalysts, homogeneous catalysts,. Reaction Graph With Catalyst.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Reaction Graph With Catalyst Reaction diagrams for a chemical process with and without a catalyst are shown below. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. To increase the rate of a reaction you need to increase the number of successful collisions. The catalyzed pathway involves a. Heterogeneous catalysts, homogeneous catalysts, and enzymes. One. Reaction Graph With Catalyst.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Reaction Graph With Catalyst A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. Lowering the activation energy of a reaction by a catalyst. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. To increase the rate of a reaction you need. Reaction Graph With Catalyst.
From www.savemyexams.co.uk
Catalysts (1.7.6) AQA A Level Chemistry Revision Notes 2017 Save My Reaction Graph With Catalyst Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. One possible way of doing this is to provide an alternative way for. In a heterogeneous reaction, the catalyst is in a different phase. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. In. Reaction Graph With Catalyst.
From saylordotorg.github.io
Catalysis Reaction Graph With Catalyst Heterogeneous catalysts, homogeneous catalysts, and enzymes. In this section, we will examine the three major classes of catalysts: To increase the rate of a reaction you need to increase the number of successful collisions. This page explains how adding a catalyst affects the rate of a reaction. In a heterogeneous reaction, the catalyst is in a different phase. In this. Reaction Graph With Catalyst.
From diagramlisthavens.z21.web.core.windows.net
Reaction Energy Diagram Transition State Reaction Graph With Catalyst To increase the rate of a reaction you need to increase the number of successful collisions. In this section, we will examine the three major classes of catalysts: In a heterogeneous reaction, the catalyst is in a different phase. Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at. Reaction Graph With Catalyst.
From shimidoon.ir
تشریح کامل قانون هس + مثال * شیمی دون Reaction Graph With Catalyst The catalyzed pathway involves a. Heterogeneous catalysts, homogeneous catalysts, and enzymes. In a heterogeneous reaction, the catalyst is in a different phase. One possible way of doing this is to provide an alternative way for. In this section, we will examine the three major classes of catalysts: A catalyst is a substance which speeds up a reaction, but is chemically. Reaction Graph With Catalyst.
From courses.lumenlearning.com
Catalysis Chemistry for Majors Reaction Graph With Catalyst In this section, we will examine the three major classes of catalysts: Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. One possible way of doing this is to provide an alternative way for. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end.. Reaction Graph With Catalyst.
From mmerevise.co.uk
Catalysts Questions and Revision MME Reaction Graph With Catalyst In a heterogeneous reaction, the catalyst is in a different phase. The catalyzed pathway involves a. To increase the rate of a reaction you need to increase the number of successful collisions. Heterogeneous catalysts, homogeneous catalysts, and enzymes. One possible way of doing this is to provide an alternative way for. In this section, we will examine the three major. Reaction Graph With Catalyst.
From www.vrogue.co
The Catalyst Chemistry Resources For Teachers vrogue.co Reaction Graph With Catalyst In this section, we will examine the three major classes of catalysts: Reaction diagrams for a chemical process with and without a catalyst are shown below. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. To increase the rate of a reaction you need to increase the number of successful collisions.. Reaction Graph With Catalyst.
From proper-cooking.info
Catalyst Chemical Reaction Reaction Graph With Catalyst A catalyst is a substance which speeds up a reaction, but is chemically unchanged at its end. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Reaction diagrams for a chemical process with and without a catalyst are shown below. Heterogeneous catalysts, homogeneous catalysts, and enzymes. In this section, we will. Reaction Graph With Catalyst.