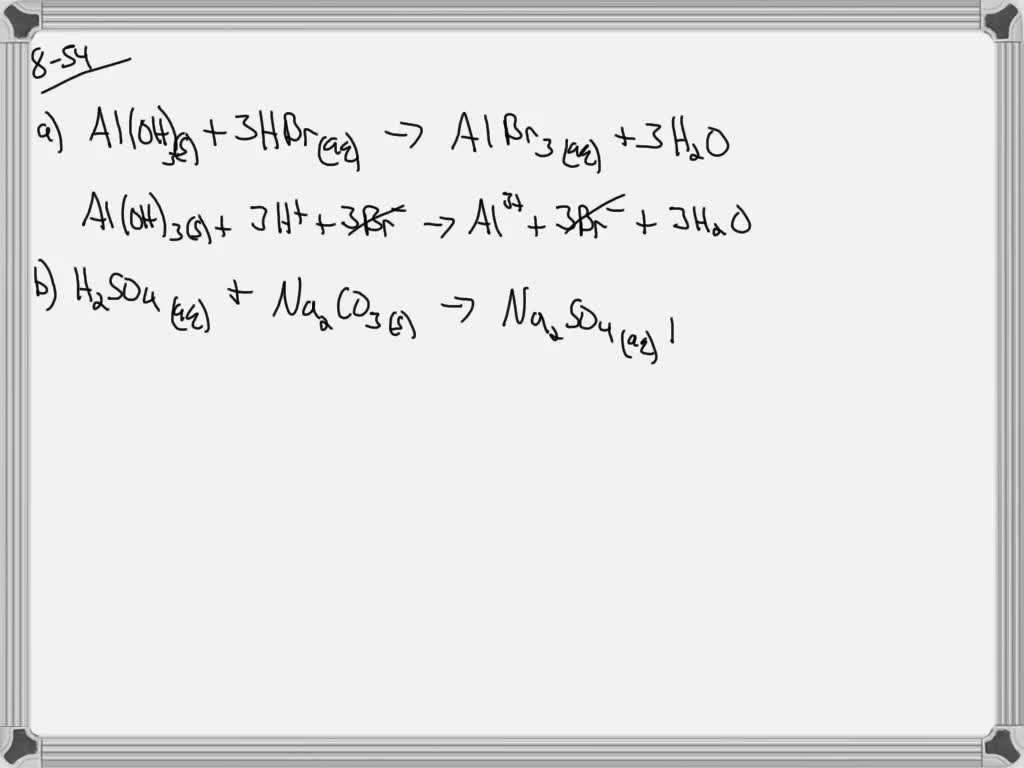Lead Nitrate Hydroxide Balanced Equation . How do you write the balanced equation. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. The products of the reaction are an aqueous solution of. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Solid silver chloride and sodium nitrate. The balanced equation will be calculated along. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. For example, silver nitrate solution reacts with sodium chloride solution. The products of the reaction are an aqueous solution of. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. enter an equation of an ionic chemical equation and press the balance button.
from www.numerade.com
How do you write the balanced equation. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. The products of the reaction are an aqueous solution of. enter an equation of an ionic chemical equation and press the balance button. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The products of the reaction are an aqueous solution of. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. Solid silver chloride and sodium nitrate.
SOLVEDand net ionic equation for the following eactions Write
Lead Nitrate Hydroxide Balanced Equation The products of the reaction are an aqueous solution of. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. Solid silver chloride and sodium nitrate. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. The products of the reaction are an aqueous solution of. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The products of the reaction are an aqueous solution of. For example, silver nitrate solution reacts with sodium chloride solution. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The balanced equation will be calculated along. How do you write the balanced equation. enter an equation of an ionic chemical equation and press the balance button. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Lead Nitrate Hydroxide Balanced Equation The products of the reaction are an aqueous solution of. The balanced equation will be calculated along. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. enter an equation of an ionic chemical equation and press the balance button. How do you write the balanced equation. write a balanced equation for the decomposition of ammonium. Lead Nitrate Hydroxide Balanced Equation.
From www.youtube.com
How to Balance Pb(NO3)2 = PbO + NO2 + O2 of Lead (II Lead Nitrate Hydroxide Balanced Equation aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The products of the reaction are an aqueous solution of. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. enter an equation of an ionic chemical equation and press the balance. Lead Nitrate Hydroxide Balanced Equation.
From www.youtube.com
How to Write the Formula for Lead (IV) nitride YouTube Lead Nitrate Hydroxide Balanced Equation enter an equation of an ionic chemical equation and press the balance button. The products of the reaction are an aqueous solution of. Solid silver chloride and sodium nitrate. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. The balanced equation will be calculated along. c 8h 18(l) +. Lead Nitrate Hydroxide Balanced Equation.
From www.chegg.com
Solved 5. Lead(II) nitrate + sodium hydroxide → Balanced Lead Nitrate Hydroxide Balanced Equation The products of the reaction are an aqueous solution of. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. For example, silver nitrate solution reacts with sodium chloride solution. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Solid silver chloride and sodium nitrate. c 8h 18(l) + 25. Lead Nitrate Hydroxide Balanced Equation.
From www.chegg.com
Solved Consider the chemical reaction that takes place Lead Nitrate Hydroxide Balanced Equation c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. enter an equation of an ionic chemical equation and press the balance button. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The products of the reaction are an aqueous solution. Lead Nitrate Hydroxide Balanced Equation.
From www.toppr.com
Write the balanced chemical equation the following reactions & identify Lead Nitrate Hydroxide Balanced Equation The balanced equation will be calculated along. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. How do you write the balanced equation. The products of the reaction are an aqueous solution of. For example, silver nitrate solution reacts with sodium chloride solution. c 8h 18(l) + 25 2 _ o 2(g). Lead Nitrate Hydroxide Balanced Equation.
From www.youtube.com
Write the balanced chemical equation for each of the following Lead Nitrate Hydroxide Balanced Equation enter an equation of an ionic chemical equation and press the balance button. Solid silver chloride and sodium nitrate. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. The balanced equation will be calculated along. The products of the reaction are an aqueous solution of. The products of the reaction are an. Lead Nitrate Hydroxide Balanced Equation.
From www.slideserve.com
PPT Balancing Chemical Reactions PowerPoint Presentation, free Lead Nitrate Hydroxide Balanced Equation aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Solid silver chloride and sodium nitrate. For example, silver nitrate solution reacts with sodium chloride solution. enter an equation of an ionic chemical equation and press the balance button. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen. Lead Nitrate Hydroxide Balanced Equation.
From www.numerade.com
SOLVED The following molecular equation represents the reaction that Lead Nitrate Hydroxide Balanced Equation c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The balanced equation will be calculated along.. Lead Nitrate Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Write the balanced net ionic equation for the reaction of Lead Nitrate Hydroxide Balanced Equation enter an equation of an ionic chemical equation and press the balance button. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. For example, silver nitrate solution reacts with sodium chloride solution. The products of the reaction are an aqueous solution of. How do you write the balanced equation. The products of the reaction are an. Lead Nitrate Hydroxide Balanced Equation.
From www.chegg.com
Solved An aqueous solution of lead(II) nitrate is mixed with Lead Nitrate Hydroxide Balanced Equation Solid silver chloride and sodium nitrate. How do you write the balanced equation. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. aqueous solutions of. Lead Nitrate Hydroxide Balanced Equation.
From www.coursehero.com
[Solved] 13_ Aqueous lead (1]) nitrate, Ph[N03)2 undergoes a double Lead Nitrate Hydroxide Balanced Equation The products of the reaction are an aqueous solution of. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. For example, silver nitrate solution reacts with sodium chloride. Lead Nitrate Hydroxide Balanced Equation.
From www.numerade.com
SOLVED 24) Aqueous sodium chloride and lead (II) nitrate solutions Lead Nitrate Hydroxide Balanced Equation lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. The products of the reaction are an aqueous solution of. The products of the reaction are an aqueous solution of. For example, silver nitrate solution reacts with sodium chloride solution. enter an equation of an ionic chemical equation and press the balance button.. Lead Nitrate Hydroxide Balanced Equation.
From dxoezqcmu.blob.core.windows.net
Lead Hydroxide Ionic Formula at Clara Lott blog Lead Nitrate Hydroxide Balanced Equation For example, silver nitrate solution reacts with sodium chloride solution. The balanced equation will be calculated along. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. How do you write the balanced equation. c. Lead Nitrate Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Write the balanced net ionic equation for the reaction of Lead Nitrate Hydroxide Balanced Equation write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. The products of the reaction are an aqueous solution of. The balanced equation will be calculated along.. Lead Nitrate Hydroxide Balanced Equation.
From www.chegg.com
Solved EACTION 10A LEAD NITRATE (Pb(NO,), AND SODIUM Lead Nitrate Hydroxide Balanced Equation aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. enter an equation of an ionic chemical equation and press the balance button. Solid silver chloride and sodium nitrate. The products of the reaction are an aqueous solution of. For example, silver nitrate solution reacts. Lead Nitrate Hydroxide Balanced Equation.
From www.youtube.com
Class10 chemistry chap1 chemical reaction (heating of lead nitrate Lead Nitrate Hydroxide Balanced Equation enter an equation of an ionic chemical equation and press the balance button. How do you write the balanced equation. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The products of the reaction are an aqueous solution of. For example, silver nitrate solution reacts with sodium chloride solution. c 8h 18(l) + 25 2. Lead Nitrate Hydroxide Balanced Equation.
From www.youtube.com
How to Balance Pb(NO3)2 = PbO + NO2 + O2 of Lead (II Lead Nitrate Hydroxide Balanced Equation Solid silver chloride and sodium nitrate. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. For example, silver nitrate solution reacts with sodium chloride solution. The products of the reaction are an. Lead Nitrate Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Write balanced chemical equations. 2) Aqueous lithium hydroxide Lead Nitrate Hydroxide Balanced Equation enter an equation of an ionic chemical equation and press the balance button. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. For example, silver nitrate solution reacts with sodium chloride solution. The balanced equation will be calculated along. How do you write the balanced equation. aqueous solutions of lead (ii). Lead Nitrate Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equation for each of the reactions Lead Nitrate Hydroxide Balanced Equation enter an equation of an ionic chemical equation and press the balance button. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. How do you write the balanced equation. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. The balanced. Lead Nitrate Hydroxide Balanced Equation.
From www.chegg.com
Solved 5 Lead(11) Nitrate + Sodium Hydroxide Balanced Lead Nitrate Hydroxide Balanced Equation write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. enter an equation of an ionic chemical equation and press the balance button. The balanced equation. Lead Nitrate Hydroxide Balanced Equation.
From www.toppr.com
(51 Write the balanced chemical equations Lead sulphate + ammonium Lead Nitrate Hydroxide Balanced Equation write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. The products of the reaction are an aqueous solution of. For example, silver nitrate solution reacts with sodium chloride solution. enter an equation of an. Lead Nitrate Hydroxide Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for HBr + NaOH = H2O + NaBr YouTube Lead Nitrate Hydroxide Balanced Equation aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. For example, silver nitrate solution reacts with sodium chloride solution. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. c 8h 18(l) + 25 2 _ o 2(g). Lead Nitrate Hydroxide Balanced Equation.
From dxoezqcmu.blob.core.windows.net
Lead Hydroxide Ionic Formula at Clara Lott blog Lead Nitrate Hydroxide Balanced Equation write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. The products of the reaction are an aqueous solution of. The products of the reaction are an aqueous solution of. The balanced equation will be calculated along. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. lead (ii). Lead Nitrate Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Lead (II) nitrate + potassium iodide Balanced Chemical Equation Lead Nitrate Hydroxide Balanced Equation aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The balanced equation will be calculated along. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. enter an equation of an ionic chemical equation and press the balance button. lead. Lead Nitrate Hydroxide Balanced Equation.
From testbook.com
Lead (II) Nitrate Formula Structure, Preparation, & Uses Lead Nitrate Hydroxide Balanced Equation enter an equation of an ionic chemical equation and press the balance button. How do you write the balanced equation. The products of the reaction are an aqueous solution of. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. aqueous solutions of. Lead Nitrate Hydroxide Balanced Equation.
From dxoojemzf.blob.core.windows.net
Sodium Hydroxide Lead Nitrate Balanced Equation at Leonard Griffin blog Lead Nitrate Hydroxide Balanced Equation write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. How do you write the balanced equation. The products of the reaction are an aqueous solution of. For example, silver nitrate solution reacts with sodium chloride solution. The products of the reaction are an aqueous solution of. aqueous solutions of lead. Lead Nitrate Hydroxide Balanced Equation.
From www.slideserve.com
PPT Chemical Reactions and Equations PowerPoint Presentation, free Lead Nitrate Hydroxide Balanced Equation aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The products of the reaction are an aqueous solution of. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. The products of the reaction are an aqueous solution of. c 8h 18(l) + 25 2 _ o 2(g). Lead Nitrate Hydroxide Balanced Equation.
From dxoojemzf.blob.core.windows.net
Sodium Hydroxide Lead Nitrate Balanced Equation at Leonard Griffin blog Lead Nitrate Hydroxide Balanced Equation For example, silver nitrate solution reacts with sodium chloride solution. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. How do you write the balanced equation. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. Solid silver chloride and sodium nitrate.. Lead Nitrate Hydroxide Balanced Equation.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Lead Nitrate Hydroxide Balanced Equation The products of the reaction are an aqueous solution of. The balanced equation will be calculated along. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide. Lead Nitrate Hydroxide Balanced Equation.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Lead Nitrate Hydroxide Balanced Equation The balanced equation will be calculated along. The products of the reaction are an aqueous solution of. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. The products. Lead Nitrate Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the reaction between aqueous lead Lead Nitrate Hydroxide Balanced Equation c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. The balanced equation will be calculated along. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. For example, silver nitrate solution reacts with sodium chloride solution. The products of the reaction are. Lead Nitrate Hydroxide Balanced Equation.
From www.numerade.com
SOLVEDand net ionic equation for the following eactions Write Lead Nitrate Hydroxide Balanced Equation write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. The balanced equation will be calculated along. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. How do you write the balanced equation.. Lead Nitrate Hydroxide Balanced Equation.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Lead Nitrate Hydroxide Balanced Equation aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The products of the reaction are an aqueous solution of. The. Lead Nitrate Hydroxide Balanced Equation.
From exohqbjbg.blob.core.windows.net
Lead Hydroxide State Of Matter at Lester Brand blog Lead Nitrate Hydroxide Balanced Equation c 8h 18(l) + 25 2 _ o 2(g) → 8co 2(g) + 9h 2o(g) 25 oxygen atoms on both reactant and product sides. enter an equation of an ionic chemical equation and press the balance button. Solid silver chloride and sodium nitrate. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen,. Lead Nitrate Hydroxide Balanced Equation.