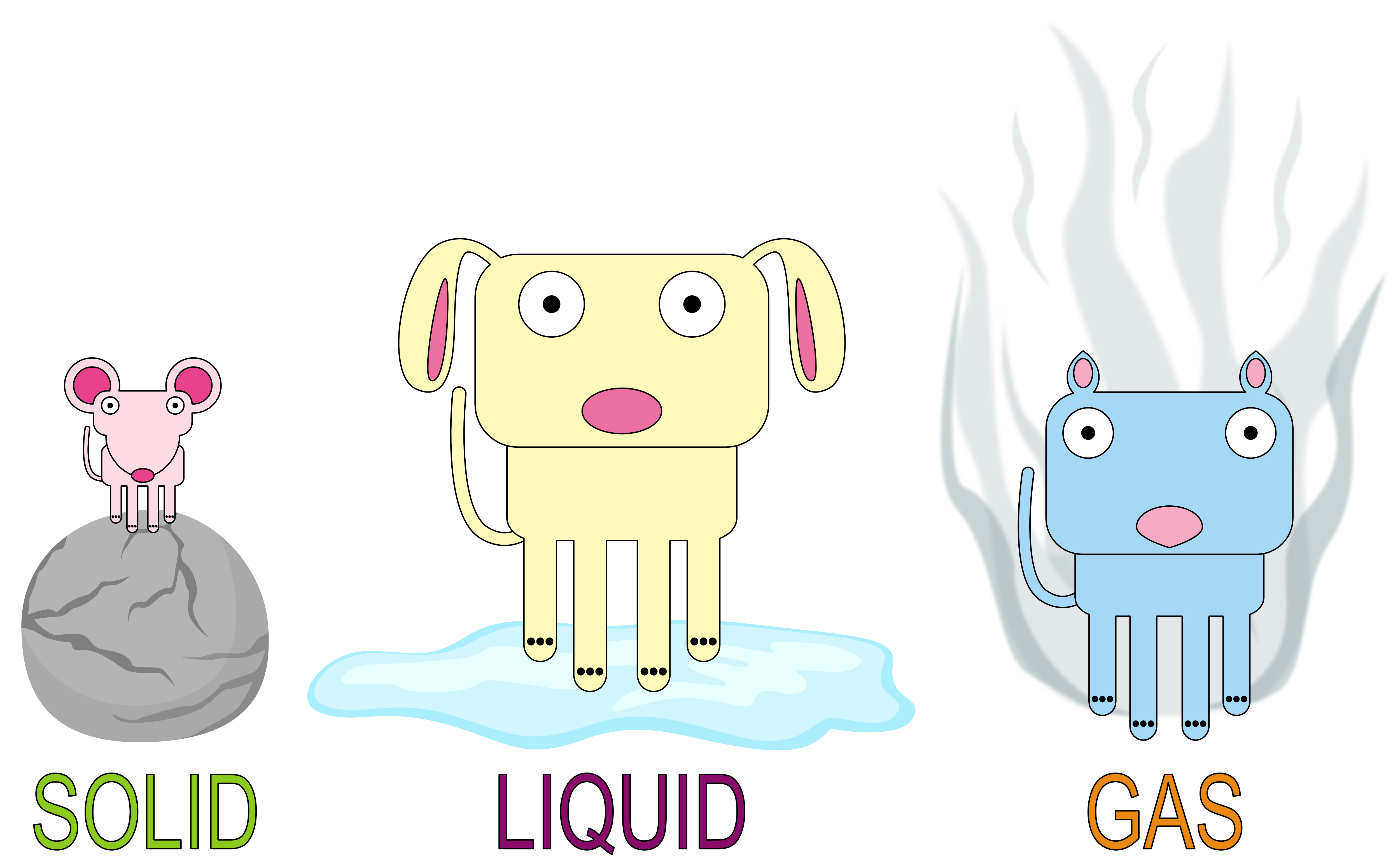
Solid Liquid I Gas . Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and condensation. solids, liquids, and gases are the three primary states of matter. most substances can exist as a solid, liquid or gas. They can change state, usually when they are heated or cooled. Solid matter is composed of tightly packed particles. You might think it's just a matter of temperature, but. solids, liquids and gases. find out what particle arrangements and movements are in solids, liquids, and gases in this bbc bitesize ks3 physics guide. matter can exist in one of three main states: solids differ from liquids and gases by the characteristic of rigidity. The molecules of solids are tightly packed because of strong intermolecular forces;. This model explains the properties. what's the difference between a solid, a liquid, and a gas? The three states of matter can be represented by the particle model.
from
You might think it's just a matter of temperature, but. This model explains the properties. Solid matter is composed of tightly packed particles. The three states of matter can be represented by the particle model. matter can exist in one of three main states: solids, liquids, and gases are the three primary states of matter. solids, liquids and gases. Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and condensation. solids differ from liquids and gases by the characteristic of rigidity. find out what particle arrangements and movements are in solids, liquids, and gases in this bbc bitesize ks3 physics guide.
Solid Liquid I Gas solids, liquids, and gases are the three primary states of matter. solids, liquids, and gases are the three primary states of matter. most substances can exist as a solid, liquid or gas. You might think it's just a matter of temperature, but. The three states of matter can be represented by the particle model. The molecules of solids are tightly packed because of strong intermolecular forces;. They can change state, usually when they are heated or cooled. Solid matter is composed of tightly packed particles. This model explains the properties. solids differ from liquids and gases by the characteristic of rigidity. solids, liquids and gases. Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and condensation. what's the difference between a solid, a liquid, and a gas? find out what particle arrangements and movements are in solids, liquids, and gases in this bbc bitesize ks3 physics guide. matter can exist in one of three main states:
From
Solid Liquid I Gas Solid matter is composed of tightly packed particles. This model explains the properties. matter can exist in one of three main states: solids, liquids, and gases are the three primary states of matter. most substances can exist as a solid, liquid or gas. The molecules of solids are tightly packed because of strong intermolecular forces;. They can. Solid Liquid I Gas.
From
Solid Liquid I Gas most substances can exist as a solid, liquid or gas. The molecules of solids are tightly packed because of strong intermolecular forces;. The three states of matter can be represented by the particle model. solids, liquids and gases. This model explains the properties. They can change state, usually when they are heated or cooled. matter can exist. Solid Liquid I Gas.
From www.madebyteachers.com
printable Solid Liquids and Gas worksheets for grade 1, 2, 3 Made By Solid Liquid I Gas most substances can exist as a solid, liquid or gas. They can change state, usually when they are heated or cooled. find out what particle arrangements and movements are in solids, liquids, and gases in this bbc bitesize ks3 physics guide. matter can exist in one of three main states: what's the difference between a solid,. Solid Liquid I Gas.
From
Solid Liquid I Gas find out what particle arrangements and movements are in solids, liquids, and gases in this bbc bitesize ks3 physics guide. solids, liquids, and gases are the three primary states of matter. You might think it's just a matter of temperature, but. The molecules of solids are tightly packed because of strong intermolecular forces;. Understanding these states is crucial. Solid Liquid I Gas.
From www.vrogue.co
States Of Matter Solid Liquid Gas Worksheet Printable vrogue.co Solid Liquid I Gas solids differ from liquids and gases by the characteristic of rigidity. The three states of matter can be represented by the particle model. matter can exist in one of three main states: what's the difference between a solid, a liquid, and a gas? They can change state, usually when they are heated or cooled. solids, liquids. Solid Liquid I Gas.
From
Solid Liquid I Gas find out what particle arrangements and movements are in solids, liquids, and gases in this bbc bitesize ks3 physics guide. solids, liquids, and gases are the three primary states of matter. The three states of matter can be represented by the particle model. most substances can exist as a solid, liquid or gas. matter can exist. Solid Liquid I Gas.
From finwise.edu.vn
Top 97+ Pictures Pictures Of Liquids Solids And Gas Sharp Solid Liquid I Gas most substances can exist as a solid, liquid or gas. matter can exist in one of three main states: Solid matter is composed of tightly packed particles. They can change state, usually when they are heated or cooled. The molecules of solids are tightly packed because of strong intermolecular forces;. This model explains the properties. solids, liquids. Solid Liquid I Gas.
From
Solid Liquid I Gas This model explains the properties. matter can exist in one of three main states: find out what particle arrangements and movements are in solids, liquids, and gases in this bbc bitesize ks3 physics guide. Solid matter is composed of tightly packed particles. solids differ from liquids and gases by the characteristic of rigidity. solids, liquids and. Solid Liquid I Gas.
From lnerpnpspu.blogspot.com
Solid Liquid Gas Diagram Solved In This Phase Diagram For Water Solid Liquid I Gas solids, liquids and gases. This model explains the properties. matter can exist in one of three main states: find out what particle arrangements and movements are in solids, liquids, and gases in this bbc bitesize ks3 physics guide. The molecules of solids are tightly packed because of strong intermolecular forces;. most substances can exist as a. Solid Liquid I Gas.
From
Solid Liquid I Gas what's the difference between a solid, a liquid, and a gas? Solid matter is composed of tightly packed particles. most substances can exist as a solid, liquid or gas. They can change state, usually when they are heated or cooled. The molecules of solids are tightly packed because of strong intermolecular forces;. solids differ from liquids and. Solid Liquid I Gas.
From www.pinterest.com
Solid, Liquid, Gas worksheet Matter worksheets, Solid liquid gas Solid Liquid I Gas Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and condensation. This model explains the properties. solids differ from liquids and gases by the characteristic of rigidity. most substances can exist as a solid, liquid or gas. Solid matter. Solid Liquid I Gas.
From
Solid Liquid I Gas Solid matter is composed of tightly packed particles. most substances can exist as a solid, liquid or gas. The molecules of solids are tightly packed because of strong intermolecular forces;. Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and. Solid Liquid I Gas.
From
Solid Liquid I Gas The molecules of solids are tightly packed because of strong intermolecular forces;. solids, liquids, and gases are the three primary states of matter. what's the difference between a solid, a liquid, and a gas? The three states of matter can be represented by the particle model. matter can exist in one of three main states: They can. Solid Liquid I Gas.
From
Solid Liquid I Gas matter can exist in one of three main states: The molecules of solids are tightly packed because of strong intermolecular forces;. solids, liquids and gases. This model explains the properties. what's the difference between a solid, a liquid, and a gas? Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you. Solid Liquid I Gas.
From saylordotorg.github.io
Solids and Liquids Solid Liquid I Gas Solid matter is composed of tightly packed particles. matter can exist in one of three main states: most substances can exist as a solid, liquid or gas. The molecules of solids are tightly packed because of strong intermolecular forces;. find out what particle arrangements and movements are in solids, liquids, and gases in this bbc bitesize ks3. Solid Liquid I Gas.
From talanewtanthony.blogspot.com
Solids Liquids and Gases TalanewtAnthony Solid Liquid I Gas You might think it's just a matter of temperature, but. Solid matter is composed of tightly packed particles. solids, liquids, and gases are the three primary states of matter. matter can exist in one of three main states: solids, liquids and gases. This model explains the properties. Understanding these states is crucial because they are fundamental concepts. Solid Liquid I Gas.
From
Solid Liquid I Gas You might think it's just a matter of temperature, but. most substances can exist as a solid, liquid or gas. The molecules of solids are tightly packed because of strong intermolecular forces;. solids, liquids and gases. This model explains the properties. They can change state, usually when they are heated or cooled. Solid matter is composed of tightly. Solid Liquid I Gas.
From
Solid Liquid I Gas find out what particle arrangements and movements are in solids, liquids, and gases in this bbc bitesize ks3 physics guide. The three states of matter can be represented by the particle model. Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing. Solid Liquid I Gas.
From
Solid Liquid I Gas solids differ from liquids and gases by the characteristic of rigidity. You might think it's just a matter of temperature, but. most substances can exist as a solid, liquid or gas. matter can exist in one of three main states: solids, liquids and gases. solids, liquids, and gases are the three primary states of matter.. Solid Liquid I Gas.
From www.youtube.com
States of Matter Solid Liquid Gas States of Matter drawing Different Solid Liquid I Gas Solid matter is composed of tightly packed particles. solids differ from liquids and gases by the characteristic of rigidity. The molecules of solids are tightly packed because of strong intermolecular forces;. matter can exist in one of three main states: most substances can exist as a solid, liquid or gas. They can change state, usually when they. Solid Liquid I Gas.
From
Solid Liquid I Gas You might think it's just a matter of temperature, but. Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and condensation. This model explains the properties. The three states of matter can be represented by the particle model. solids, liquids,. Solid Liquid I Gas.
From www.e-streetlight.com
Solid Liquid Gas Worksheet Solid Liquid I Gas solids, liquids, and gases are the three primary states of matter. You might think it's just a matter of temperature, but. matter can exist in one of three main states: The molecules of solids are tightly packed because of strong intermolecular forces;. solids, liquids and gases. solids differ from liquids and gases by the characteristic of. Solid Liquid I Gas.
From ar.inspiredpencil.com
Liquids Solids And Gases For Kids Solid Liquid I Gas You might think it's just a matter of temperature, but. They can change state, usually when they are heated or cooled. This model explains the properties. what's the difference between a solid, a liquid, and a gas? Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real. Solid Liquid I Gas.
From
Solid Liquid I Gas The three states of matter can be represented by the particle model. find out what particle arrangements and movements are in solids, liquids, and gases in this bbc bitesize ks3 physics guide. solids, liquids, and gases are the three primary states of matter. Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and. Solid Liquid I Gas.
From
Solid Liquid I Gas most substances can exist as a solid, liquid or gas. You might think it's just a matter of temperature, but. solids, liquids and gases. Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and condensation. what's the difference. Solid Liquid I Gas.
From
Solid Liquid I Gas The molecules of solids are tightly packed because of strong intermolecular forces;. solids differ from liquids and gases by the characteristic of rigidity. solids, liquids and gases. The three states of matter can be represented by the particle model. Solid matter is composed of tightly packed particles. They can change state, usually when they are heated or cooled.. Solid Liquid I Gas.
From
Solid Liquid I Gas Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and condensation. solids, liquids, and gases are the three primary states of matter. matter can exist in one of three main states: The molecules of solids are tightly packed because. Solid Liquid I Gas.
From
Solid Liquid I Gas matter can exist in one of three main states: The three states of matter can be represented by the particle model. Solid matter is composed of tightly packed particles. This model explains the properties. They can change state, usually when they are heated or cooled. solids, liquids, and gases are the three primary states of matter. Understanding these. Solid Liquid I Gas.
From www.snexplores.org
Explainer What are the different states of matter? Solid Liquid I Gas They can change state, usually when they are heated or cooled. most substances can exist as a solid, liquid or gas. The molecules of solids are tightly packed because of strong intermolecular forces;. You might think it's just a matter of temperature, but. solids, liquids and gases. matter can exist in one of three main states: Solid. Solid Liquid I Gas.
From studymertterrifyxr.z21.web.core.windows.net
Science Solid Liquid Gas Worksheets Solid Liquid I Gas solids, liquids, and gases are the three primary states of matter. Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and condensation. This model explains the properties. You might think it's just a matter of temperature, but. solids, liquids. Solid Liquid I Gas.
From
Solid Liquid I Gas They can change state, usually when they are heated or cooled. This model explains the properties. Solid matter is composed of tightly packed particles. solids, liquids and gases. solids, liquids, and gases are the three primary states of matter. most substances can exist as a solid, liquid or gas. Understanding these states is crucial because they are. Solid Liquid I Gas.
From lessonlibraryhexane.z13.web.core.windows.net
Picture Solid Liquid Gas Solid Liquid I Gas matter can exist in one of three main states: Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and condensation. most substances can exist as a solid, liquid or gas. This model explains the properties. The molecules of solids. Solid Liquid I Gas.
From www.artofit.org
Solids liquids and gases activities Artofit Solid Liquid I Gas solids differ from liquids and gases by the characteristic of rigidity. what's the difference between a solid, a liquid, and a gas? solids, liquids, and gases are the three primary states of matter. solids, liquids and gases. find out what particle arrangements and movements are in solids, liquids, and gases in this bbc bitesize ks3. Solid Liquid I Gas.
From exohfkhuv.blob.core.windows.net
Solids Liquids And Gases Class 4 Questions And Answers at William Solid Liquid I Gas Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and condensation. You might think it's just a matter of temperature, but. matter can exist in one of three main states: most substances can exist as a solid, liquid or. Solid Liquid I Gas.
From japaneseclass.jp
Images of SOLID JapaneseClass.jp Solid Liquid I Gas This model explains the properties. solids differ from liquids and gases by the characteristic of rigidity. solids, liquids, and gases are the three primary states of matter. find out what particle arrangements and movements are in solids, liquids, and gases in this bbc bitesize ks3 physics guide. most substances can exist as a solid, liquid or. Solid Liquid I Gas.